* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download + H 2 O(l)
Metallic bonding wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Geochemistry wikipedia , lookup
Oxidation state wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Citric acid cycle wikipedia , lookup
Click chemistry wikipedia , lookup
Stoichiometry wikipedia , lookup
Coordination complex wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Liquid–liquid extraction wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Water splitting wikipedia , lookup
History of electrochemistry wikipedia , lookup
Chemical reaction wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Biochemistry wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Electrolysis of water wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Electrochemistry wikipedia , lookup
Metalloprotein wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
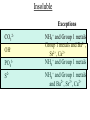
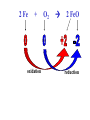
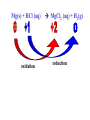
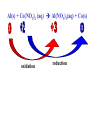
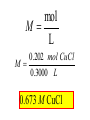
Reactions in aqueous solutions • Aqueous solution: – Solution in which water is the solvent (dissolving agent). • 3 major types of chemical processes of aqueous solutions: – Precipitation reactions – Acid-base reactions – Redox reactions • Solution: –Homogenous mixture of 2 or more substances. • Solvent: –Dissolving medium, usually present in greater quantity. • Solute: –The other substance(s) in the solution. • Electrolyte: – A substance whose aqueous solution forms ions; conducts electricity. – Ionic compounds. • Nonelectrolyte: – Substance that does not form ions in an aqueous solution; poor conductor. – Molecular compounds. • Ionic compounds in water: – Dissociate into its component ions. dissolving_NaCl_probe.swf Do not get to wrapped up in the difference between the terms ionization and dissociation. Consider them to mean the same thing, the separation of a substances ions. Equations showing ionization or dissociation NaCl (aq) Na (aq) Cl (aq) CuCl2 (aq) Cu 2 (aq) 2Cl (aq) 3 Na3PO4 (aq) 3Na (aq) PO 4 (aq) Molecular compounds in H2O Molecular compounds – nonmetal + nonmetal Structural integrity of molecule is usually maintained meaning no ions form (C12H22O11) Exception: Some molecular solutes interact with water to form ions. These would be electrolytes. Examples: Acids HCl, H2C3O2 Ammonia NH3 dissolving_sugar_probe.swf Strong Electrolytes • Exists in solution completely or almost completely as ions. • All ionic compounds and a few molecular compounds.(Ex: Strong Acids) HCl (aq) NaCl( s) H Na (aq) (aq) Cl Cl (aq) (aq) Weak Electrolytes • Molecular compounds that produce a small concentration of ions when dissolved in H2O. Ex: Acetic acid (HC2H3O2) only slightly ionizes when dissolved in water. HC2H3O2(aq) H+(aq)+ C2H3O2-(aq) Weak acids are better conductors if they are dilute, as you will see in lab. Explain. • Reactions that result in an insoluble product. • Insoluble: –Substance with solubility less than 0.01 mol/L –Water molecules cannot overcome the attraction between the ions. KI (aq) + Pb(NO3)2 (aq) PbI2(s) + KNO3 (aq) You must be able to determine whether a substance is soluble in water by simple examination of the chemical formula. To do so, you must memorize how specific polyatomic ions act in water. Not as hard as it sounds. We will focus mainly on 10 anions. This will give you the tools to predict the solubility of many compounds. Solubility of Ionic Compounds • All acetates and nitrates are soluble in water. • All ionic compounds of alkali metals and ammonium are soluble. – (1A goes AWAY) • Solubility rules are on your reference sheet. Soluble Exceptions Cl- Ag+, Hg22+, Pb2+ Acetate ion C2H3O2- None Br- Ag+, Hg22+, Pb2+ I- Ag+, Hg22+, Pb2+ NO3- None SO42- Sr2+, Ba2+, Hg22+, Pb2+ Insoluble Exceptions CO32OH- PO43S2- NH4+ and Group 1 metals Group 1 metals and Ba2+, Sr2+, Ca2+ NH4+ and Group 1 metals NH4+ and Group 1 metals and Ba2+, Sr2+, Ca2+ Equation Types • Molecular Pb( NO3 )2 2KI( aq) PbI 2( s ) 2KNO3aq) • Complete Ionic 2 ( aq) Pb 2 NO( aq3 ) 2K ( aq) 2I ( aq) PbI 2( s ) 2K • Net ionic equation 2 ( aq) Pb 2I ( aq) PbI2( s ) ( aq) 3( aq) 2 NO Ionic Equations 2 2 NO 2K 2I Pb(aq PbI 2 K 2 NO 3(aq) (aq) 2( s ) ( aq) 3( aq) ) (aq) •Those ions that appear on both sides of a complete ionic equation are known as Spectator Ions. •Net ionic equations do not include spectator ions. • Exchange Reactions • Metathesis reactions • Double displacement • Double replacement AX BY AY BX AgNO3( aq) KCl( aq) AgCl( s ) KNO3( aq) Writing Net Ionic Equations 1) Write a balanced molecular equation. 2) Rewrite the equation showing ions of strong electrolytes only. 3) Identify and cancel all spectator ions. Acid-Base Reactions Acids: • Ionize in H2O, causes increase in H+ ions. • H+ ions are bare protons. • Acids are proton donors. All this acid rain is killing my complexion! • Monoprotic Acids: (HCl, HNO3) –Acids that can only yield one H+ per molecule upon ionization. HCl H+ + Cl- Diprotic Acids: (H2SO4) Ionization occurs in 2 steps. H 2 SO4( aq) H 4( aq) HSO ( aq) H 4( aq) HSO ( aq) 2 4( aq) SO Only the first ionization is complete. Is HF a weak or strong acid? weak acid Although it is a weak acid, this acid is extremely reactive because of the F- ion. Must be kept in special polypropylene container because it eats through glass. Used to etch glass. Has caused major accidents in lab. • Substances that increase the OH- when added to water. (NaOH) • NH3 is a base. In water it accepts an H+ ion from HOH, leaving an OH- in solution. – NH3 is a weak electrolyte – About 1% ionizes to form NH4+/OH- Strong acids and bases • Acids and bases that ionize completely in solution are strong acids and bases. • Those that only ionize partially are weak acids and bases. • You must memorize these. Strong Acids Hydrochloric Acid – HCl Hydrobromic Acid – HBr Hydroiodic Acid – HI Nitric Acid – HNO3 Sulfuric Acid – H2SO4 Chloric Acid – HClO3 Perchloric Acid – HClO4 Strong Bases All group 1 Metal Hydroxides (LiOH, NaOH, KOH, RbOH, CsOH) Heavy Group 2 Metal Hydroxides Ca(OH)2, Sr(OH)2, Ba(OH)2 Once you memorize the strong acids and bases, you will have enough information to determine if a substance is a strong or weak electrolyte. Example problems: KF Na3PO4 NH3 CH3CH2OH HCl NO2 HC2H3O2 CH4 NH4Cl CH3Cl strong electrolyte strong electrolyte weak electrolyte nonelectrolyte strong electrolyte nonelectrolyte weak electrolyte nonelectrolyte strong electrolyte nonelectrolyte Acid + Base Neutralization • Products of a neutralization reaction have none of the properties of an acid or a base. • An acid reacts with a metal hydroxide to form a salt plus water. Neutralization Reactions • Acid + Base (Metal Hydroxide) Salt + Water • HCl(aq) + NaOH(aq) NaCl(aq) + H2O(l) • H+ + Cl- + Na+ + OH- Na+ + Cl- + H2O(l) H+ + OH- H2O(l) Write the net ionic equation for the following reaction. It might help to first write the molecular equation, and then the complete ionic equation, followed by the net ionic equation. Potassium Hydroxide + Sulfuric Acid • Ionic equation: 2K(aq) 2OH (aq) 2H (aq) SO4(2aq) 2 HOH (l ) 2K(aq) SO4(2aq) • Net Ionic equation: H ( aq) OH ( aq) H 2O(l ) Neutralization Reaction of Weak Acid *Remember, only strong electrolytes are written as ions.* Acetic Acid + Sodium Hydroxide HC2H3O2(aq) + NaOH(aq) NaC2H3O2(aq) + H2O(l) Weak acid strong base soluble salt water HC2H3O2 + Na+ + OH- Na+ + C2H3O2- + H2O(l) HC2H3O2(aq) + OH (aq) C2H3O2 (aq)+ H2O(l) Acid/Base Rx’s with gas formation • Other bases besides OH- react with H+ to form molecular compounds.Two common -2 -2 bases are CO3 and S . • Carbonates and bicarbonates react with acid to form CO2. Hydrochloric acid + Sodium Sulfide 2HCl (aq) + Na2S(aq) H2S(g) + 2NaCl(aq) 2H+ (aq) + S2-(aq) H2S(g) Hydrochloric acid + Sodium Hydrogen Carbonate HCl (aq) + NaHCO3(aq) NaCl(aq) + H2CO3(aq) H2CO3(aq) H2O(l) + CO2(g) HCl (aq) + NaHCO3(aq) NaCl(aq) + H2O(l) + CO2(g) H+ (aq) + HCO3-(aq) H2O(l) + CO2(g) Reactions in which electrons are transferred between substances Use of Oxidation numbers in determining redox reactions is basically a bookkeeping method for keeping track of electrons You must be able to identify an oxidation-reduction reaction. But first, we must learn the rules for assigning oxidation #’s to different species. Rules for oxidation numbers 1) Atoms in elemental form are 0. 2) Monatomic ion; charge of the ion is its oxidation number. 3) Nonmetals; usually negative numbers. a.) oxygen = -2 unless a peroxide = -1 b.) Hydrogen +1 with nonmetals, -1 with metals c.) Halogens (-1) unless bonded to oxygen (+) in a polyatomic ion (Ex: ClO3-; Cl = +5) 4) Sum of oxidation numbers must = 0 5) Most electronegative (furthest to right and up) element gets a negative charge. See pages 128 – 129 for more on this. 1) Atoms in elemental form are 0. Examples Ag Pb Cl2 O2 Oxidation # = 0 for 7 diatomic elements and for all other elements when by themselves. 2) Monatomic ion-charge of the ion is its oxidation number. Examples AgCl Ag = +1 PbI2 Pb = +2 Fe2O3 Fe = +3 Cl = -1 I = -1 O = -2 3) Nonmetals; usually negative numbers. a.) oxygen = -2 unless a peroxide = -1 b.) Hydrogen +1 with nonmetals, -1 with metals c.) Halogens (-1) unless bonded to oxygen (+) in a polyatomic ion (Ex: ClO3-; Cl = +5) PbO H2S KI Examples oxygen = -2 Na2O2 hydrogen = +1 NaH iodine = -1 KIO2 oxygen = -1 hydrogen = -1 iodine = + 3 Determine Oxidation # of element red element in each of the following: MnO2 +4 Br2 0 KMnO4 HClO4 +7 +7 BrO2- H2SO4 +3 +6 BrO3- PO33- +5 +3 CaH2 -1 SO42- +6 Na2S -2 Mg(NO3)2 +5 Again, oxidation reduction reactions occur when there is a transfer of electrons from one species to another in a reaction. • If one reactant gains electrons another must lose electrons. • Reduction is always accompanied by oxidation. Oxidation-Reduction Reactions • An atom that becomes more positively charged is oxidized. –This is due to loss of e-. • The gain of electrons by an atom is called reduction. Two mnemonics for remembering which substance is undergoing oxidation and which is undergoing reduction? OIL -- RIG Oxidation Involves Loss -- Reduction Involves Gain “Leo the lion says Ger” Loss of electrons oxidation -- Gain of electrons reduction Many metals react with O2 in the air to form metal oxides. Metals lose electrons to oxygen. 2 Fe + O2 2 FeO As Fe is oxidized (loses e-), oxygen is reduced (gains e-). Reduction is gain 2 Fe + oxidation O2 2 FeO reduction Oxidation of metals by acids and salts • Reaction of a metal with either an acid or metal salt follows general form of: A + BX AX +B • Single displacement reaction +2+6-2 0 +2+6-2 0 CuSO4(aq) + Zn(s) ZnSO4(aq) + Cu(s) Reduced Oxidized What are the products? What are the charges on each species? What is oxidized and what is reduced? For the following reactants: 1) Write the reaction that occurs. 2) Identify what is being oxidized and reduced. Magnesium + Hydrochloric Acid Aluminum + Cobalt(II) Nitrate Mg(s) + HCl (aq) MgCl2 (aq) + H2(g) oxidation reduction Al(s) + Co(NO3)2 (aq) Al(NO3)3(aq) + Co(s) oxidation reduction Types of Redox Reactions • Combination (synthesis) • Decomposition • Displacement – hydrogen, metal, halogen • Disproportionation (When an element is simultaneously oxidized and reduced). • Ex: H2O2 H2O + O2 Activity Series • List of metals in order of decreasing ease of oxidation. • Alkali and alkaline earth metals are at the top. (active metals) • Gold, Silver, Platinum, and palladium are considered to be (noble metals) because they resist oxidation. Using activity series to predict reactions • Activity series can be used to predict reactions between metals and metal salts or acids. • Any metal listed on the series can be oxidized by the ions of elements below it on the list. Using activity series of metals, which metals from the list below can be oxidized by H+? Ni Al Cu Pb Ag Mg Au This should be a review before handing out All reaction types worksheet. Must edit all reaction type worksheet. It has to many weak acids reacting with strong bases, which is confusing for students. Make sure most have reactions (see note in folder on handout). Chemical Reaction Types • Decomposition • Synthesis • Single Replacement • Precipitation • Neutralization • Combustion Types of Redox Reactions Types of Double Replacement Reactions What are the 7 Diatomic Elements H2 – hydrogen N2 – nitrogen O2 – oxygen F2 - fluorine Cl2 – chlorine Br2 – bromine I2 – Iodine Synthesis A + B AB Examples H2 (g) + O2 (g) H2O (g) Mg (s) + O2 (g) MgO (s) Na (s) + Cl2 (g) NaCl (s) Decomposition AB A + B Examples NaCl (s) Na (s) + Cl2 (g) elec KClO3 (s) KCl (s) + O2 (g) Single Replacement A + BC AC + B Examples Na (s) + HOH (l) NaOH (aq) + H2 (g) sodium replaces hydrogen in water Cl2 (g) + NaBr (aq) Br2 (l) + NaCl (aq) chlorine replaces bromine in sodium bromide Double Replacement (Metathesis) AB + CD AD + BC Examples AgNO3 (aq) + NaCl (aq) AgCl (s) + NaNO3 (aq) HCl (aq) + NaOH (aq) NaCl (aq) + H2O (l) NH4Cl (aq) + NaOH (aq) NH3 (g) + H2O (l) + NaCl (aq) Blue color for the products represents the driving force which allows the chemical reaction to occur. Combustion hydrocarbon + oxygen carbon dioxide + water Examples CH4 (g) + O2 (g) CO2 (g) + H2O (l) C3H8 (g) + O2 (g) CO2 (g) + H2O (l) CH3OH (g) + O2 (g) CO2 (g) + H2O (l) CO2 + H2O Neutralization Reactions Strong Acid + Strong Base Salt + Water Example HCl (aq) + NaOH (aq) NaCl (aq) + H2O (l) H+ + + + Cl + Na + OH Na + Cl + H2O(l) H+ + OH- H2O Concentration (Molarity) • Concentration — the amount of solute per unit of solution. • Molarity (M) — expresses concentration: Moles of solute (M ) Volume of solution in Liters Calculate the molarity of a solution that contains 20.0g copper(I) chloride and has a total volume of 300.0 mL. mol M L Must have the correct units. Given: 20.0 grams CuCl; 300.0 ml solution Need: Moles CuCl; L of solution 20.0 g CuCl 1 mol CuCl 99.0 g CuCl 300.0 mL 1 L 1000 mL = 0.202 mol CuCl = 0.3000 L mol M L 0.202 mol CuCl M 0.3000 L 0.673 M CuCl How many grams of NaCl are needed to make 2.5 L of 0.20 molar solution? Given: 0.20 M solution; 2.5 L solution Need: grams of NaCl mol (L) (L) M L Must calculate # of moles, and then convert it into grams. mol = ML Mol = ML Mol = 0.20 mol NaCl x 2.5 L = 0.50 mol NaCl L 0.50 mol NaCl 55.84 g NaCl 1 = mol NaCl 28 g NaCl Dilution of Stock Solutions • Chemicals are purchased in concentrated form. They need to be diluted for most lab use. • Formula for dilution: Mi Vi = Mf Vf How much stock (12 M) HCl (aq) is required to make 200.0 mL of 3M HCl (aq)? Mi Vi = Mf Vf Mi = 12 M Vf = 200.0 mL Mf = 3 M Vi = ? mL Must rearrange equation above to solve for initial volume . Vi M fVf Mi (3.0M )( 200 .0ml ) Vi 50. mL (12 M ) Measure 150 mL of water in a beaker. Slowly add 50.0 mL of 12 M HCl for a final volume of 200.0 mL. Two things to note: 1) Always add concentrated acid to water, and not the reverse to avoid unwanted splashing due to the heat generated. 2) When diluting a solution, the amount of solute doesn’t change, only the final volume. If diluting a solution other than acids, start with initial volume of concentrated solution, and then dilute with distilled water until you have the desired volume. You try one! We want to prepare 500. mL of 1.00 M acetic acid from a 17.5 M stock solution of acetic acid. What volume of the stock solution is required? Mi Vi = Mf Vf Mi = 17.5 M Vf = 500. mL Mf = 1.00 M Vi = ? mL M fVf Vi Mi (1.00 M )(500 .0ml ) Vi 28.6 mL (17 .5M ) Pour 471.4 mL of distilled water into a beaker. Slowly pour the 28.6 ml of acid into the water and swirl. Fill the container with distilled water to 500. mL. There may be times when you must consider the concentration of ions in a solution. (You must consider the subscripts for this) MgCl2 Mg2+ + 2Cl- In a solution of 0.25 M MgCl2 you have: M of Mg2+ = 0.25 M M of Cl- = 2 x 0.25 M = 0.50 M What is the concentration of each ion in the following? 0.15 Na3P M of Na+ = 0.45 M M of P3- = 0.15 M Titrations • Determining the concentration of an unknown solution. • Use a 2nd solution of known concentration (standard solution) that undergoes a reaction with the unknown solution. • Use the ratios in the balanced equation along with the M = mol/L equation to determine molarity of unknown. • The point at which the two solutions are stoichiometrically equal is known as the equivalence point. –The reaction is complete and no excess reactant is present. –How do we know when this occurs during the reaction? • In acid base reactions dyes known as indicators are used. – Phenolphthalein is colorless in acid solution, and pink in basic solution. – End point is reached when a drop of the base remains pink. There is no acid for this drop to react with and the solution is now basic.