* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Ch.5
Biochemistry wikipedia , lookup
Supramolecular catalysis wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Chemical potential wikipedia , lookup
Chemical industry wikipedia , lookup
Catalytic reforming wikipedia , lookup
Host–guest chemistry wikipedia , lookup
Safety data sheet wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Water splitting wikipedia , lookup
Strengthening mechanisms of materials wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Electrolysis of water wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Marcus theory wikipedia , lookup
History of chemistry wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Chemical bond wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Isotopic labeling wikipedia , lookup
Discodermolide wikipedia , lookup
George S. Hammond wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Electrochemistry wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Molecular dynamics wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Chemical reaction wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Transition state theory wikipedia , lookup
Click chemistry wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Rate equation wikipedia , lookup
Process chemistry wikipedia , lookup
History of molecular theory wikipedia , lookup
Chemical equations describe
CHEMICAL REACTIONS.
During a chemical reaction, the ways in
which atoms are joined together are
changed.
OLD bonds are broken and NEW bonds
are formed as REACTANTS are converted
into PRODUCTS.
A reaction STARTS with substances known as REACTANTS
(always written on the LEFT side of the equation).
The substances that are FORMED during a reaction are
known as PRODUCTS (always written on the RIGHT side of
the equation).
Chemical Equations
Chemical Equations are used (as chem.
shorthand) to represent what is
occurring during a chemical reaction.
Ex.: Butane burns in oxygen to produce
carbon dioxide gas and water vapor.
C4H10 + O2 --->
CO2 + H2O
∆
Why Balance Equations?
When 9.386 g Ca reacts completely with 7.514 g S, 16.90 g of
CaS are formed.
Ca(s) + S(s) ---> CaS(s)
Balancing Equations
Make sure the chemical
formulas are CORRECT (you
cannot change the correct
formula of a substance).
I2 Br2 Cl2 F2 O2 N2
H2
I Bring Clay For Our New House
Use COEFFICIENTS (numbers
in front of formulas) to change
the number of atoms of an
element.
Coefficients represent
MULTIPLES of the formulas
Balance the following
N2 (g) +
H2 (g) --->
FeCl3 (s) --->
Fe(s) +
NH3 (g)
Cl2
(g)
NaOCl + KI + HC2H3O2 ---> I2 + NaCl + KC2H3O2 + H2O
Balance the following
C3H8 + O2 --> CO2 + H2O
C3H6 + O2 --> CO2 + H2O
Types of Chemical Reactions
Combination
Decomposition
Single Replacement
Double Replacement
Neutralization
Combustion
A + B --> AB
AB --> A + B
A + BC --> AC + B
AC + BD --> AD + BC
A + O2 --> AO
CxHy + O2 --> x CO2 + y/2 H2O
RedOx
Measuring MATTER
Technique
- Necessary information
Counting
- Avogadro’s #
Weighing
- Molar Mass
Measuring Volume - Molar Volume
Types of Chemical Particles
Atoms - represented by the symbol of an
element (C, Ag, N, Na, Cl, Fe, Pb, S, etc.)
Molecules - represented by multiple symbol(s)
of NONMETAL atoms/elements (CO2, H2O, O2,
CH4, etc.)
Ions - represented by the symbol/formula of an
ion
Formula Units - represented by the formula of
an IONIC compound (NaCl, AgNO3, Fe2O3,
etc.)
1 mole = 6.02 x 1023 particles
(a conversion factor)
Avogadro’s Number = 6.02 x 1023
This number can be determined
experimentally several ways:
Measurement of crystal structure
Ti - body-centered unit cell (2 atoms/unit cell)
# of atoms/mol
2 Ti atoms
1 unit cell
47.88g
1 cm 3
= 6.02 x 1023atoms
unit cell
(3.306 x 10-8cm)3
1 mol
4.401 g Ti
MOLAR MASS
The number of grams of a substance
equivalent to the sum of all its average atomic
mass units (amu) is known as the molar
mass.
One mole of particles is equal to its
molar mass in grams.
MOLAR VOLUME
The volume, 22.4 L, of any gas at STP
is known as the molar volume.
STP = Standard Temperature and
Pressure
273 K (O˚C, 32 ˚F) and 1 atm (101.3 kPa, 760 mm Hg, 29.92 in Hg)
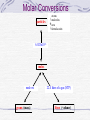
Molar Conversions
particles
atoms
molecules
ions
formula units
{
6.023x10 23
moles
mole wt.
grams (mass)
22.4 liters of a gas (STP)
liters (volume)
Molar Conversions
STOICHIOMETRY -think recipes!
Balanced chemical equations can be used to
predict the QUANTITATIVE amounts of
REACTANTS and PRODUCTS.
N2(g) +
Particles
Molecules
Atoms
Moles
Mass
3H2 (g) --->
2NH3 (g)
N2(g) + 3H2 (g) ---> 2NH3 (g)
How many moles of hydrogen will react with 0.00326 mol N2?
How many molecules of ammonia are produced when 4.55 x 1018 molecules of
hydrogen react?
How many atoms of hydrogen are in the ammonia produced?
How many grams of nitrogen are required to react with 75.8 g hydrogen?
Stoichiometry
Use the stoichiometric mole ratio to convert from
moles of one substance to moles of another
substance within the reaction
C4H10 +
O2 --->
CO2 + H2O
How many moles of water are produced when 0.48
moles of butane, C4H10, react?
How many molecules of butane are needed to
produce 12.00 grams of H2O?
Stoichiometry, cont.
In the lab, we determine the masses of different
substances, rather than moles, and therefore must be
able to convert from grams of one substance in a
reaction to grams of another.
•
NaOCl +
•
How many grams of iodine are produced when 0.35 g of
potassium iodide react?
KI +
HC2H3O2 --->
I2 +
NaCl +
KC2H3O2 +
H2O
I2 (aq) +
Na2S2O3(aq) -->
Na2S4O6(aq) +
NaI
How many grams of sodium iodide are produced
when 0.203 g of iodine react with excess
Na2S2O3?
Which reactant limits the amount of product that
can be made? Why?
Limiting Reactants
The product(s) of a reaction is/are
limited by how much of each reactant
is present (available) in the reaction.
Two types of reactants
Limiting - this is the reactant you run
out of first!
Excess - at the end of the reaction there
will be some of this reactant left over
(excess:-)).
Combustion of Magnesium
Mg(s) + O2(g in air) --> MgO(s)
How much magnesium oxide can be produced when 1.085 g Mg
burns in air?
Determine the limiting and excess reactants
Calculate the theoretical yield from the limiting reactant
Mg(s) + O2(g in air) --> MgO(s)
Molar Mass
208.3g/mol
76.1 g/mol
BaCl2 + NH4SCN --> Ba(SCN) 2 + NH4Cl
34.5 g BaCl2 react with 44.3 g NH4SCN.
How much NH4Cl can be produced (theoretical yield)?
STEP 1: Determine the LR and ER! (use mole ratio)
STEP 2: Determine the Theoretical Yield from LR
Experimental Reaction Yield
Balanced equations can be used to
calculate the amount of product that
will form during a reaction - called
the THEORETICAL YIELD
The amount of product that actually
forms during a chemical reaction is
called the ACTUAL YIELD
The actual yield is often less than the
theoretical yield.
Percent Yield
The percent yield is the ratio of the
actual yield compared to the
theoretical yield, converted to a
percent.
actual yield
% YIELD = --------------------- x 100
theoretical yield
N2(g) + H2(g) --->
NH3 (g)
0.075 g N2 react with 0.0095 g H2 to produce 0.051 g NH3.
Which reactant is the limiting reactant?
Which reactant is in excess?
How much ammonia should be produced?
What is the percent yield for this reaction?
Mole Ratios in Chemical Formulas
The Empirical (Simplest) Formula is a ratio of atoms
in the compound (this is equivalent to the mole ratio
of atoms).
Ex. If 3.10 g Fe reacts with chlorine to make 9.01 g
of a compound, what is the simplest formula of the
compound?
Mole Ratios in Chemical Formulas
For hydrated compounds, the mole ratio of water to
the compound is expressed in the formula.
Ex. If 2.00 g of a copper (II) sulfate hydrate is heated
and the mass of the anhydrate is 1.28 g, what is the
formula of the hydrate?
Oxidation & Reduction
RedOx
Reactions that involve the transfer of electrons
between “particles” are known as RedOx rxns.
Oxidation is the loss of electron(s) from an atom.
Reduction is the gain of electron(s) by an atom.
OIL RIG
Examples:
Rusting; Batteries; Antiseptics; Combustion of
Hydrocarbons; Reactions in Biochemical Pathways
Chemistry 104
Quiz #6
1.
How many grams do 8.5 x 1025 molecules of water
weigh?
2.
Balance the equation:
C6H14 + O2 CO2 + H2O
3.
For the reaction:
N2H4 + 2H2O2 N2 + 4H2O
How many grams of dinitrogen tetrahydride are
needed to form 20.0 g dihydrogen monoxide?
4.
If 18.0 g hydrogen peroxide react with the amount of dinitrogen
tetrahydride determined in Q.#3 and produces 15.6 g water, what
is the percent yield?