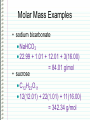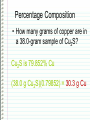* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Changing Matter
Biochemistry wikipedia , lookup
Host–guest chemistry wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Chemical reaction wikipedia , lookup
Click chemistry wikipedia , lookup
History of chemistry wikipedia , lookup
Self-assembled monolayer wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Isotopic labeling wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Transition state theory wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Extended periodic table wikipedia , lookup
Chemical bond wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Rate equation wikipedia , lookup
Process chemistry wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Molecular dynamics wikipedia , lookup
Thermometric titration wikipedia , lookup
Computational chemistry wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Implicit solvation wikipedia , lookup
History of molecular theory wikipedia , lookup
Atomic theory wikipedia , lookup
Geometrical frustration wikipedia , lookup
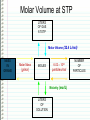
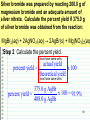
Changing Matter • Matter can be changed two ways: –Physically • Physical reaction • Physical change –Chemically • Chemical reaction • Chemical change Physical Changes • Do NOT CHANGE THE TYPE OF MATTER – Nothing new or different is formed – Could be a change in: • • • • • • Mass Size Volume Density Change in state Color Shape Examples of Physical Changes • • • • Boiling, vaporization…. Any state change Dissolving Breaking Making a mixture – 2 or more types of matter (substances) mixed together • Not in specific amounts • Can be separated physically Chemical Changes • Atoms have electrons arranged in energy levels or energy shells • Electrons in the last (outermost) shell are called valence electrons • Valence electrons let atoms bond with other atoms – Ionic bonding • Gaining or losing electrons – Covalent bonding • Sharing electrons Chemical Changes • Atoms that bond form molecules – May be the same type (nonmetals) of atom or, – Different types (metal + nonmetal) of atoms • Different types compounds Chemical Changes • Molecules can bond and “unbond” – Atoms can re-arranged in different combinations – For example: CaCO3 (1 atom Ca, 1 atom C, 3 atoms O) Add heat to re-arranged the atoms: CaO CO2 Chemical Changes • Evidence of a chemical reaction – Formation of gas – Formation of precipitate – Change in color – Change in energy • Endothermic – Absorbs heat energy (gets cold) • Exothermic – Releases heat energy (gets hot) Chemical Changes • Chemical reactions can be represented by equations CaCO3 CaO + CO2 Reactants Products Chemical Changes • Atoms are re-arranged, NOT created or destroyed – Law of Conservation of Matter – Law of Conservation of Mass Chemical Changes • Matter is conserved type of atoms does not change – Nothing is created or destroyed • Mass is conserved amount of atoms cannot change – Nothing is created or destroyed Chemical Changes • To show conservation of mass Balance equations – Make sure there are the same number of each type of atom in the products and in the reactants Balancing Equations The equation for the burning of methane gas in oxygen is: CH4 + 2 O2 → CO2 + 2 H2O Subscript Coefficient Shows # of atoms Shows # of molecules Balancing Equations • No subscript or coefficient is understood to be 1 CH4 + 2 O2 → CO2 + 2 H2O = C 1H 4 + 2 O 2 → C 1 O 2 + 2 H 2O 1 1C 4H 4O 1C 4H 4O Periodic Properties Post Lab • Periodic Trends affect properties because of Zeff – Ionization energy • Energy required to remove an electron – Electronegativity • Ability to attract electrons – Atomic Radius • Distance of the valence electrons to the nucleus Other Periodic Trends • Groups have similar properties – Valence electrons • Members of the same representative family have the same number of valence electrons – Reactivity • Because they have the same number of electrons, they react similarly. – CaCl2, MgCl2, SrCl2, etc. – Density = mass/volume • % error = I actual – theoretical I theoretical Sn = 7.265 Pb = 11.34 Si = 2.33 Ge = 5.323 Solubility Trends • Common in Double Replacement Rxns Double Replacement Rxns • Two compounds react to form two new compounds – Metal replaces metal – Remember basic formula writing rules Ex: Ca(NO3)2 + Na2CO3 CaCO3 + 2 NaNO3 ppt Some Types of Chemical Reactions Type of Reaction Definition Synthesis Two or more elements or compounds combine to make a more complex substance Decomposition Compounds break down into simpler substances Single Replacement Occurs when one element replaces another one in a compound Double Replacement Occurs when different atoms in two different compounds trade places Equation A + B → AB AB → A + B AB + C → AC + B AB + CD → AC + BD Identifying Chemical Reactions S = Synthesis ____ P + D = Decomposition O2 → P4O10 ____ HgO → Hg ____ Cl2 + + SR = Single Replacement DR = Double Replacement ____ Mg + O2 O2 NaBr → NaCl + Br2 → ____ Al2O3 MgO → Al + O2 ____ H2 + N2 → NH3 MOLAR MASS Molar Mass is shown below the element symbol on the Periodic Table. Units: grams mole Use molar mass to convert between: Number of Moles Mass (grams) Number of Molecules or Atoms Molar Mass Examples • sodium bicarbonate NaHCO3 22.99 + 1.01 + 12.01 + 3(16.00) • sucrose = 84.01 g/mol C12H22O11 12(12.01) + 22(1.01) + 11(16.00) = 342.34 g/mol Divide the mass (in grams) by Molar Mass to get the number of moles. MASS in GRAMS MOLAR MASS grams/mol Multiply the moles by the Molar Mass to get the mass (in grams). MOLES Divide the number of molecules or atoms by Avogadro’s Constant to get the number of moles. AVOGADRO’S CONSTANT 6.022x1023 particles/mol Multiply the number of moles by Avogadro’s Constant to get the number of atoms or molecules. MOLECULES ATOMS Molar Conversion Examples • How many moles of carbon are in 26 g of carbon? 26 g C 1 mol C = 2.2 mol C 12.01 g C Molar Conversion Examples • How many molecules are in 2.50 moles of C12H22O11? 23 6.02 10 2.50 mol molecules = 1.51 1024 molecules 1 mol C12H22O11 Molar Conversion Examples • Find the mass of 2.1 1024 molecules of NaHCO3. 2.1 1024 molecules 1 mol 84.01 g 6.02 1023 1 mol molecules = 290 g NaHCO3 Sample Problems – Moles and Atoms • Determine the number of atoms present in 2.50 moles of strontium. • Convert 5.01 x 1024 atoms of strontium to moles of strontium. Sample Problems – Moles and Mass • Determine the mass in grams of 2.50 moles of strontium. • Determine the number of moles represented by 943.5 grams of strontium. Sample Problems – Moles, Atoms, Mass • Determine the mass in grams of (exactly) 5 atoms of strontium. • Determine the number of atoms represented by 43.5 grams of strontium. Percentage Composition • the percentage by mass of each element in a compound mass of element % composition 100 total mass Percentage Composition • Find the % composition of Cu2S. 127.10 g Cu 79.852% %Cu = 100 = Cu 159.17 g Cu2S %S = 32.07 g S 159.17 g Cu2S 100 = 20.15% S Percentage Composition • Find the percentage composition of a sample that is 28 g Fe and 8.0 g O. 28 g 100 = 78% Fe %Fe = 36 g %O = 8.0 g 36 g 100 = 22% O Percentage Composition • How many grams of copper are in a 38.0-gram sample of Cu2S? Cu2S is 79.852% Cu (38.0 g Cu2S)(0.79852) = 30.3 g Cu Percentage Composition • Find the mass percentage of water in calcium chloride dihydrate, CaCl2•2H2O? %H2O = 36.04 g 100 = 24.51% H2O 147.02 g Empirical Formula • Smallest whole number ratio of atoms in a compound C 2H 6 reduce subscripts CH3 Empirical Formula 1. Find mass (or %) of each element. 2. Find moles of each element. 3. Divide moles by the smallest # to find subscripts. 4. When necessary, multiply subscripts by 2, 3, or 4 to get whole #’s. Empirical Formula • Find the empirical formula for a sample of 25.9% N and 74.1% O. 25.9 g 1 mol = 1.85 mol N =1N 1.85 mol 14.01 g 74.1 g 1 mol = 4.63 mol O = 2.5 O 16.00 g 1.85 mol Empirical Formula N1O2.5 Need to make the subscripts whole numbers multiply by 2 N2O5 Molecular Formula • “True Formula” - the actual number of atoms in a compound empirical formula CH3 ? molecular formula C2H6 Molecular Formula 1. Find the empirical formula. 2. Find the empirical formula mass. 3. Divide the molecular mass by the empirical mass. 4. Multiply each subscript by the answer from step 3. MF mass n EF mass EF n Molecular Formula • The empirical formula for ethylene is CH2. Find the molecular formula if the molecular mass is 28.1 g/mol? empirical mass = 14.03 g/mol 28.1 g/mol 14.03 g/mol = 2.00 (CH2)2 C2H4 Using the Basics… The empirical formula of a compound is found to be P2O5. The molar mass of the compound is 284 grams/mole. What is the molecular formula for the compound? Proportional Relationships • Stoichiometry – mass relationships between substances in a chemical reaction – based on the mole ratio • Mole Ratio – indicated by coefficients in a balanced equation 2 Mg + O2 2 MgO N2 + 3H2 2NH3 1 mol 3 mol 2 mol 1 mol N 2 3 mol H 2 43 N2 + 3H2 2NH3 1 mol 3 mol 2 mol 3 mol H 2 2 mol NH 3 44 Stoichiometry Steps 1. Write a balanced equation. 2. Identify known & unknown. 3. Line up conversion factors. •– Mole ratio - moles molesmoles moles – Molar mass - moles grams – Molarity moles liters soln – Molar volume - moles liters gas Core step in all stoichiometry problems!! 4. Check answer. Molar Volume at STP 1 mol of a gas=22.4 L at STP Standard Temperature & Pressure 0°C and 1 atm Molar Volume at STP LITERS OF GAS AT STP Molar Volume (22.4 L/mol) MASS IN GRAMS Molar Mass MOLES (g/mol) 6.02 1023 particles/mol Molarity (mol/L) LITERS OF SOLUTION NUMBER OF PARTICLES Mole-Mole Calculations 48 Phosphoric Acid • Phosphoric acid (H3PO4) is one of the most widely produced industrial chemicals in the world. • Most of the world’s phosphoric acid is produced by the wet process which involves the reaction of phosphate rock, Ca5(PO4)3F, with sulfuric acid (H2SO4). Ca5(PO4)3F(s) + 5H2SO4 3H3PO4 + HF + 5CaSO4 49 Calculate the number of moles of phosphoric acid (H3PO4) formed by the reaction of 10 moles of sulfuric acid (H2SO4). Ca5(PO4)3F + 5H2SO4 3H3PO4 + HF + 5CaSO4 1 mol 5 mol 3 mol 1 mol 5 mol Step 1 Moles starting substance: 10.0 mol H2SO4 Step 2 The conversion needed is moles H2SO4 moles H3PO4 Mole Ratio 3 mol H 3PO4 10 mol H 2SO4 x = 6 mol H3PO4 5 mol H 2SO4 50 Calculate the number of moles of sulfuric acid (H2SO4) that react when 10 moles of Ca5(PO4)3 react. Ca5(PO4)3F + 5H2SO4 3H3PO4 + HF + 5CaSO4 1 mol 5 mol 3 mol 1 mol 5 mol Step 1 The starting substance is 10.0 mol Ca5(PO4)3F Step 2 The conversion needed is moles Ca5(PO4)3F moles H2SO4 Mole Ratio 5 mol H 2SO4 10 mol Ca 5 (PO4 )3F x = 50 mol H 2SO4 1 mol Ca 5 (PO4 )3F 51 Stoichiometry Problems • How many moles of KClO3 must decompose in order to produce 9 moles of oxygen gas? 2KClO3 2KCl + 3O2 ? mol 9 mol O2 2 mol KClO3 3 mol O2 9 mol = 6 mol KClO3 Mole-Mass Calculations 53 Calculate the number of moles of H2SO4 necessary to yield 784 g of H3PO4. Ca5(PO4)3F+ 5H2SO4 3H3PO4 + HF + 5CaSO4 Method 1 Step by Step Step 1 The starting substance is 784 grams of H3PO4. Step 2 Convert grams of H3PO4 to moles of H3PO4. 1 mol H 3PO4 784 g H 3PO4 = 8.00 mol H 3PO4 98.0 g H 3PO4 Step 3 Convert moles of H3PO4 to moles of H2SO4 by the mole-ratio method. 5 mol H 2SO4 8.00 mol H 3PO4 = 13.3 mol H 2SO4 3 mol H 3PO4 54 Mole Ratio Calculate the number of moles of H2SO4 necessary to yield 784 g of H3PO4 Ca5(PO4)3F+ 5H2SO4 3H3PO4 + HF + 5CaSO4 Method 2 Continuous The conversion needed is grams H3PO4 moles H3PO4 moles H2SO4 Mole Ratio 1 mol H 3PO4 784 g H 3PO4 98.0 g H 3PO4 5 mol H 2SO4 = 13.3 mol H 2SO4 3mol H 3PO4 55 Stoichiometry Problems • How many grams of KClO3 are req’d to produce 9.00 L of O2 at STP? 2KClO3 2KCl + 3O2 ?g 9.00 L 9.00 L O2 1 mol O2 2 mol 122.55 KClO3 g KClO3 22.4 L O2 3 mol O2 1 mol KClO3 = 32.8 g KClO3 Mass-Mass Calculations 57 Calculate the number of grams of NH3 formed by the reaction of 112 grams of H2. N2 + 3H2 2NH3 Method 1 Step by Step Step 1 The starting substance is 112 grams of H2. Convert 112 g of H2 to moles. grams moles 1 mol H 2 112 g H 2 55.4 moles H 2 2.02 g H 2 Step 2 Calculate the moles of NH3 by the mole ratio method. 2 mol NH 3 55.4 moles H 2 = 36.9 moles NH 3 3 mol H 2 58 Calculate the number of grams of NH3 formed by the reaction of 112 grams of H2. N2 + 3H2 2NH3 Method 1 Step by Step Step 3 Convert moles NH3 to grams NH3. moles grams 17.0 g NH 3 36.9 moles NH 3 = 629 g NH 3 1 mol NH 3 59 Calculate the number of grams of NH3 formed by the reaction of 112 grams of H2. N2 + 3H2 2NH3 Method 2 Continuous grams H2 moles H2 moles NH3 grams NH3 1 mol H 2 2 mol NH 3 17.0 g NH 3 112 g H 2 = 629 g NH 3 2.02 g H 2 3 mol H 2 1 mol NH 3 60 Stoichiometry Problems • How many grams of silver will be formed from 12.0 g copper? Cu + 2AgNO3 2Ag + Cu(NO3)2 12.0 g ?g 12.0 1 mol 2 mol 107.87 g Cu Cu Ag g Ag = 40.7 g 63.55 1 mol 1 mol Ag g Cu Cu Ag Stoichiometry Problems • How many grams of Cu are required to react with 1.5 L of 0.10M AgNO3? Cu + 2AgNO3 2Ag + Cu(NO3)2 ?g 1.5L 0.10M 1.5 .10 mol 1 mol 63.55 L AgNO3 Cu g Cu 1L = 4.8 g 2 mol 1 mol Cu AgNO3 Cu Limiting Reactant 63 • The limiting reactant is one of the reactants in a chemical reaction. • It is called the limiting reactant because the amount of it present is insufficient to react with the amounts of other reactants that are present. • The limiting reactant limits the amount of product that can be formed. 64 How manyFrom bicycles From eight three wheels From pedal four fourassemblies frames four can be assembled bikes three can be bikes constructed. bikes cancan be be constructed. constructed. from the parts shown? The limiting part is the number of pedal assemblies. 65 9.2 H2 + Cl2 2HCl + 4 molecules Cl2 can form 8 molecules HCl 7 molecules H2 can form 14 molecules HCl 9.3 Cl is the limiting reactant 3 molecules of H2 remain 2 H2 is in excess 66 Steps Used to Determine the Limiting Reactant 67 1. Calculate the amount of product (moles or grams, as needed) formed from each reactant. 2. Determine which reactant is limiting. (The reactant that gives the least amount of product is the limiting reactant; the other reactant is in excess. 3. Calculate the amount of the other reactant required to react with the limiting reactant, then subtract this amount from the starting quantity of the reactant. This gives the amount of the substance that remains unreacted. 68 Examples 69 How many moles of HCl can be produced by reacting 4.0 mol H2 and 3.5 mol Cl2? Which compound is the limiting reactant? H2 + Cl2 → 2HCl Step 1 Calculate the moles of HCl that can form from each reactant. 2 mol HCl 4.0 mol H 2 8.0 mol HCl 1 mol H 2 3.5 mol Cl2 2 mol HCl 7.0 mol HCl 1 mol Cl 2 Step 2 Determine the limiting reactant. The limiting reactant is Cl2 because it 70 produces less HCl than H2. How many moles of silver bromide (AgBr) can be formed when solutions containing 50.0 g of MgBr2 and 100.0 g of AgNO3 are mixed together? How many grams of the excess reactant remain unreacted? MgBr2(aq) + 2AgNO3 (aq) → 2AgBr(s) + Mg(NO3)2(aq) Step 1 Calculate the grams of AgBr that can form from each reactant. The conversion needed is g reactant → mol reactant → mol AgBr → g AgBr 1 mol MgBr2 2 mol AgBr 187.8 g AgBr 102 g AgBr 50.0 g MgBr 2 184.1 g MgBr2 1 mol MgBr2 1 mol AgBr 1 mol AgNO3 2 mol AgBr 187.8 g AgBr 110.5 g AgBr 100.0 g AgNO3 71 169.9 g AgNO3 2 mol AgNO3 1 mol AgBr How many moles of silver bromide (AgBr) can be formed when solutions containing 50.0 g of MgBr2 and 100.0 g of AgNO3 are mixed together? How many grams of the excess reactant remain unreacted? MgBr2(aq) + 2AgNO3 (aq) → 2AgBr(s) + Mg(NO3)2(aq) Step 2 Determine the limiting reactant. The limiting reactant is MgBr2 because it forms less Ag Br. 1 mol MgBr2 2 mol AgBr 187.8 g AgBr 102 g AgBr 50.0 g MgBr 2 184.1 g MgBr2 1 mol MgBr2 1 mol AgBr 1 mol AgNO3 2 mol AgBr 187.8 g AgBr 110.5 g AgBr 100.0 g AgNO3 72 169.9 g AgNO3 2 mol AgNO3 1 mol AgBr How many grams of the excess reactant (AgNO3) remain unreacted? MgBr2(aq) + 2AgNO3 (aq) → 2AgBr(s) + Mg(NO3)2(aq) Step 3 Calculate the grams of unreacted AgNO3. First calculate the number of grams of AgNO3 that will react with 50 g of MgBr2. The conversion needed is g MgBr2 → mol MgBr2 → mol AgNO3 → g AgNO3 1 mol MgBr2 2 mol AgNO3 169.9 g AgNO3 50.0 g MgBr2 92.3 g AgNO3 184.1 g MgBr2 1 mol MgBr2 1 mol AgNO3 The amount of MgBr2 that remains is 100.0 g AgNO3 92.3 g AgNO3 = 7.7 g AgNO733 Reaction Yield 74 The quantities of products calculated from equations represent the maximum yield (100%) of product according to the reaction represented by the equation. 75 Many reactions fail to give a 100% yield of product. This occurs because of side reactions and the fact that many reactions are reversible. 76 • The theoretical yield of a reaction is the calculated amount of product that can be obtained from a given amount of reactant. • The actual yield is the amount of product finally obtained from a given amount of reactant. 77 • The percent yield of a reaction is the ratio of the actual yield to the theoretical yield multiplied by 100. actual yield x 100 = percent yield theoretical yield 78 Silver bromide was prepared by reacting 200.0 g of magnesium bromide and an adequate amount of silver nitrate. Calculate the percent yield if 375.0 g of silver bromide was obtained from the reaction: MgBr2(aq) + 2AgNO3 (aq) → 2AgBr(s) + Mg(NO3)2(aq Step 1 Determine the theoretical yield by calculating the grams of AgBr that can be formed. The conversion needed is g MgBr2 → mol MgBr2 → mol AgBr → g AgBr 1 mol MgBr2 200.0 g MgBr 2 184.1 g MgBr 2 2 mol AgBr 187.8 g AgBr 408.0 g AgBr 1 mol MgBr2 1 mol AgBr 79 Silver bromide was prepared by reacting 200.0 g of magnesium bromide and an adequate amount of silver nitrate. Calculate the percent yield if 375.0 g of silver bromide was obtained from the reaction: MgBr2(aq) + 2AgNO3 (aq) → 2AgBr(s) + Mg(NO3)2(aq Step 2 Calculate the percent yield. must have same units actual yield percent yield = x 100 theoretical yield must have same units 375.0 g AgBr x 100 = 91.9% percent yield = 408.0 g AgBr 80