* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 3
Self-assembled monolayer wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Rate equation wikipedia , lookup
Transition state theory wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Biochemistry wikipedia , lookup
Electron configuration wikipedia , lookup
Abundance of the chemical elements wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Process chemistry wikipedia , lookup
Strengthening mechanisms of materials wikipedia , lookup
Chemical element wikipedia , lookup
Metalloprotein wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Isotopic labeling wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
History of chemistry wikipedia , lookup
Computational chemistry wikipedia , lookup
Thermometric titration wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Chemical bond wikipedia , lookup
Extended periodic table wikipedia , lookup
Molecular dynamics wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Implicit solvation wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Geometrical frustration wikipedia , lookup
History of molecular theory wikipedia , lookup
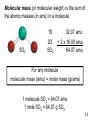
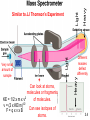
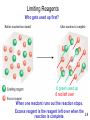
Mass Relationships in Chemical Reactions Chapter 3 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Micro World atoms & molecules Macro World grams Atomic mass is the mass of an atom in atomic mass units (amu) By definition: 1 atom 12C “weighs” 12 amu On this scale 1H = 1.008 amu 16O = 16.00 amu 3.1 Natural lithium is: 7.42% 6Li (6.015 amu) 92.58% 7Li (7.016 amu) Average atomic mass of lithium: 7.42 x 6.015 + 92.58 x 7.016 = 6.941 amu 100 3.1 Average atomic mass (6.941) The mole (mol) is the amount of a substance that contains as many elementary entities as there are atoms in exactly 12.00 grams of 12C 1 mol = NA = 6.0221367 x 1023 Avogadro’s number (NA) 3.2 How many atoms of He are in 5.36 moles of He? 23 atoms He 6.022 x 10 5.36 mol He x = 1 mol He 3.23 x 1024 atoms He How many atoms of O are in 5.36 moles of O2? 5.36 mol O2 6.022 x 1023 molecules O2 2 atoms O = x x molecules 1 O2 molecule 1 mol O2 molecules 6.46 x 1024 atoms O eggs Molar mass is the mass of 1 mole of shoes in grams marbles atoms 1 mole 12C atoms = 6.022 x 1023 atoms = 12.00 g 1 12C atom = 12.00 amu 1 mole 12C atoms = 12.00 g 12C 1 mole lithium atoms = 6.941 g of Li For any element atomic mass (amu) = molar mass (grams) 3.2 One Mole of: S C Hg Cu Fe 3.2 1 12C atom 12.00 g 1.66 x 10-24 g x = 23 12 12.00 amu 6.022 x 10 C atoms 1 amu 1 amu = 1.66 x 10-24 g or 1 g = 6.022 x 1023 amu M = molar mass in g/mol NA = Avogadro’s number 3.2 Do You Understand Molar Mass? How many atoms are in 0.551 g of potassium (K) ? 1 mol K = 39.10 g K 1 mol K = 6.022 x 1023 atoms K 1 mol K 6.022 x 1023 atoms K 0.551 g K x x = 1 mol K 39.10 g K 8.49 x 1021 atoms K 3.2 Molecular mass (or molecular weight) is the sum of the atomic masses (in amu) in a molecule. 1S SO2 2O SO2 32.07 amu + 2 x 16.00 amu 64.07 amu For any molecule molecular mass (amu) = molar mass (grams) 1 molecule SO2 = 64.07 amu 1 mole SO2 = 64.07 g SO2 3.3 Molecular mass (or molecular weight) is the sum of the atomic masses (in amu) in a molecule. HNO3 1H 1N 1.008 amu 14.0067 amu 3O + 3 x 16.00 amu HNO3 63.01 amu 1 molecule HNO3 = 63.01 amu 1 mole HNO3 = 63.01 g HNO3 3.3 Ba(NO3)2 1Ba 2N 137.327 amu 2 x 14.0067 amu 6 O + 6 x 16.00 amu Ba(NO3)2 261.33 amu Ba 137.327 amu - OR - 2 NO3 + 2 x 62.00 amu Ba(NO3)2 261.33 amu 1 Ba(NO3)2 formula unit = 261.33 amu 1 mole Ba(NO3)2 = 261.33 g Ba(NO3)2 How many atoms are in 1.00 mol of Ba(NO3)2 ? 1.00 mol Ba(NO3)2 23 Ba(NO )2 6.022 x 10 3 x 1 mol Ba(NO3)2 9 atoms = x 1 Ba(NO3)2 5.42 x 1024 atoms 3.3 Do You Understand Molecular Mass? How many H atoms are in 72.5 g of C3H8O ? 1 mol C3H8O = (3 x 12) + (8 x 1) + 16 = 60 g C3H8O 1 mol C3H8O molecules = 8 mol H atoms 1 mol H = 6.022 x 1023 atoms H 1 mol C3H8O 8 mol H atoms 6.022 x 1023 H atoms 72.5 g C3H8O x x x = 1 mol C3H8O 1 mol H atoms 60 g C3H8O 5.82 x 1024 atoms H 3.3 + Can look at atoms, molecules or fragments of molecules. KE = 1/2 x m x v2 v = (2 x KE/m)1/2 Can see isotopes of F=qxvxB atoms. Heavy Different masses deflect differently Heavy Light Very small amount of sample Light Similar to JJ Thomson’s Experiment 3.4 Percent composition of an element in a compound = n x molar mass of element x 100% molar mass of compound n is the number of moles of the element in 1 mole of the compound 2 x (12.01 g) x 100% = 52.14% 46.07 g 6 x (1.008 g) %H = x 100% = 13.13% 46.07 g 1 x (16.00 g) %O = x 100% = 34.73% 46.07 g %C = C2H6O 52.14% + 13.13% + 34.73% = 100.0% 3.5 Empirical Formulas Weight % of Elements Ratio of Elements 1 - Start with weight percent of elements (make sure they total to 100%). 2 - Assume 100 gram sample and convert weight percent of each element to grams of each element. 3 - Convert to moles of each element using atomic weights. 4 - Calculate relative ratio of elements by dividing by the smallest number of moles. 5 - Multiply to get rid of any fractions, if necessary. When in doubt, convert to moles !!! A compound is found to contain: 20.2 wt. % Al and 79.8 wt. % Cl. What is its empirical formula? 1 - Make sure total is 100%. 20.2 % + 79.8 % = 100.0% 2 - Convert weight percent of each element to grams of each element. 20.2 g Al and 79.8 g Cl 3 - Convert to moles of each element using atomic weights. 20.2 g Al = 0.749 mol Al 79.8 g Cl = 2.25 mol Cl 26.98 g/mol Al 35.345 g/mol Cl 4 - Calculate relative ratio of elements by dividing by the smallest number of moles. => 0.749 mol Al is smaller 0.749 mol Al 0.749 mol Al = 1.00 2.25 mol Cl 0.749 mol Al => AlCl3 = 3.01mol Cl / mol Al Round to 3 Combust 11.5 g ethanol Collect 22.0 g CO2 and 13.5 g H2O g CO2 mol CO2 mol C gC 6.0 g C = 0.5 mol C g H2O mol H2O mol H gH 1.5 g H = 1.5 mol H g of O = g of sample – (g of C + g of H) 4.0 g O = 0.25 mol O Empirical formula C0.5H1.5O0.25 Divide by smallest subscript (0.25) Empirical formula C2H6O 3.6 A process in which one or more substances is changed into one or more new substances is a chemical reaction A chemical equation uses chemical symbols to show what happens during a chemical reaction 3 ways of representing the reaction of H2 with O2 to form H2O reactants products 3.7 reactants products Starting materials Substances formed aA+bB plus cC +dD to yield plus Conservation of Mass Must have the same number of atoms on each side of the reaction !! 3.7 How to “Read” Chemical Equations 2 Mg + O2 2 MgO 2 atoms Mg + 1 molecule O2 makes 2 formula units MgO 2 moles Mg + 1 mole O2 makes 2 moles MgO 48.6 grams Mg + 32.0 grams O2 makes 80.6 g MgO IS NOT 2 grams Mg + 1 gram O2 makes 2 g MgO 3.7 Balancing Chemical Equations 1. Write the correct formula(s) for the reactants on the left side and the correct formula(s) for the product(s) on the right side of the equation. Ethane reacts with oxygen to form carbon dioxide and water C2H6 + O2 CO2 + H2O 2. Change the numbers in front of the formulas (coefficients) to make the number of atoms of each element the same on both sides of the equation. Do not change the subscripts. 2C2H6 NOT C4H12 3.7 Balancing Chemical Equations 3. Start by balancing those elements that appear in only one reactant and one product. C2H6 + O2 2 carbon on left C2H6 + O2 6 hydrogen on left C2H6 + O2 CO2 + H2O start with C or H but not O 1 carbon on right multiply CO2 by 2 2CO2 + H2O 2 hydrogen on right 2CO2 + 3H2O multiply H2O by 3 3.7 Balancing Chemical Equations 4. Balance those elements that appear in two or more reactants or products. C2H6 + O2 2 oxygen on left 2CO2 + 3H2O multiply O2 by 7 2 4 oxygen + 3 oxygen = 7 oxygen (3x1) on right (2x2) remove fraction multiply both sides by 2 4. Remove all fractions (generally by multiplying everything by 2) and reduce all stoichiometric coefficients to try to get one equal to 1 (generally by dividing everything by 2). C2H6 + 7 O2 2 2C2H6 + 7O2 2CO2 + 3H2O 4CO2 + 6H2O 3.7 Balancing Chemical Equations 5. Check to make sure that you have the same number of each type of atom on both sides of the equation. 2C2H6 + 7O2 4CO2 + 6H2O 14 O (7 x 2) 12 H (2 x 6) 4 C (2 x 2) 14 O (4 x 2 + 6) 12 H (6 x 2) 4C Always show a check of your solution on a quiz or exam !! Reactants 4C 12 H 14 O Products 4C 12 H 14 O 3.7 Balancing Chemical Equations 1. Write the correct formula(s) for the reactants on the left side and the correct formula(s) for the product(s) on the right side of the equation. 2. Change the numbers in front of the formulas (coefficients) to make the number of atoms of each element the same on both sides of the equation. Do not change the subscripts. 3. Start by balancing those elements that appear in only one reactant and one product. 4. Balance those elements that appear in two or more reactants or products. 4. Remove all fractions (generally by multiplying everything by 2) and reduce all stoichiometric coefficients to try to get one equal to 1 (generally by dividing everything by 2). 5. Check to make sure that you have the same number of each type of atom on both sides of the equation. Stoichiometry Quantitative study of chemical reactions. How much in ? => How much out ? You may have used these principles baking cookies, doing carpentry, ……….. Chemicals react in ratios of moles, NOT in weight ratios !! When in doubt, convert to moles !!! CH4 + 2 O2 CO2 + 2 H2O Get a set of relationships between all reactants and products: 1 mol CH4 = 2 mol O2 = 1 mol CO2 = 2 mol H2O These are exact conversions !!! Mass Changes in Chemical Reactions When in doubt, convert to moles !!! 1. Write balanced chemical equation 2. Convert quantities of known substances into moles 3. Use coefficients in balanced equation to calculate the number of moles of the sought quantity 4. Convert moles of sought quantity into desired units 3.8 How many mole of CO2 can be generated from 10.31 moles of O2? CH4 + 2 O2 CO2 + 2 H2O 1 mol CH4 = 2 mol O2 = 1 mol CO2 = 2 mol H2O 10.31 mol O2 x 1 mol CO2 2 mol O2 = 5.155 mol CO2 How many grams of CH4 are needed to produce 3.85 mol of CO2? 3.85 mol CO2 x 1 mol CH4 x 16.04 g CH4 1 mol CO2 1 mol CH4 = 61.8 g CH4 MW CH4 = 12.01 + 4(1.008) = 16.04 g / mol CH4 Methanol burns in air according to the equation 2CH3OH + 3O2 2CO2 + 4H2O If 209 g of methanol are used up in the combustion, what mass of water is produced? grams CH3OH moles CH3OH molar mass CH3OH 209 g CH3OH x moles H2O grams H2O molar mass coefficients H2O chemical equation 4 mol H2O 18.0 g H2O 1 mol CH3OH = x x 32.0 g CH3OH 2 mol CH3OH 1 mol H2O 235 g H2O 3.8 Limiting Reagents Who gets used up first? 6 green used up 6 red left over When one reactant runs out the reaction stops. Excess reagent is the reagent left over when the 3.9 reaction is complete. How many mole of NH3 can be generated by mixing 2.35 mol N2 and 6.50 mol of H2? N2 + 3 H2 2 NH3 1 mol N2 = 3 mol H2 = 2 mol NH3 2.35 mol N2 x 2 mol NH3 1 mol N2 = 4.70 mol NH3 6.50 mol H2 x 2 mol NH3 3 mol H2 = 4.33 mol NH3 Lower number H2 is limiting reagent and the amount of NH3 that can be generated is 4.33 mol Do You Understand Limiting Reagents? In one process, 124 g of Al are reacted with 601 g of Fe2O3 2Al + Fe2O3 Al2O3 + 2Fe Calculate the mass of Al2O3 formed. g Al mol Al g Fe2O3 124 g Al x mol Fe2O3 needed OR mol Al needed mol Fe2O3 1 mol Al 27.0 g Al x g Fe2O3 needed 1 mol Fe2O3 2 mol Al Start with 124 g Al 160. g Fe2O3 = x 1 mol Fe2O3 g Al needed 367 g Fe2O3 need 367 g Fe2O3 Have more Fe2O3 (601 g) so Al is limiting reagent 3.9 Use limiting reagent (Al) to calculate amount of product that can be formed. g Al mol Al mol Al2O3 2Al + Fe2O3 124 g Al x 1 mol Al 27.0 g Al x 1 mol Al2O3 2 mol Al g Al2O3 Al2O3 + 2Fe 102. g Al2O3 = x 1 mol Al2O3 234 g Al2O3 3.9 Theoretical Yield is the amount of product that would result if all the limiting reagent reacted. Actual Yield is the amount of product actually obtained from a reaction. % Yield = Actual Yield x 100 % Theoretical Yield Did you get as much as you thought you were going to get ??? Can calculate using weight or moles? NOTE: Law of conservation of mass dictates that % Yield < 100 % 3.10 If the theoreticall yield is 4.33 mol NH3 and 3.62 mol NH3 was recovered, what is the % yield ? N2 + 3 H2 % Yield = 2 NH3 Actual Yield x 100 % Theoretical Yield 3.62 mol NH3 4.33 mol NH3 X 100 % = 83.6 % If 26.5 g Mg was reacted with an excess of O2 and a 75.2 % yield was achieved, how much MgO was recovered ? 2 Mg + O2 26.5 g Mg x 1 mol Mg 24.30 g Mg 2 MgO = 1.09 mol Mg 2 mol Mg = 2 mol MgO 1.09 mol Mg x 2 mol MgO = 1.09 mol MgO theoretical yield 2 mol Mg 1.09 mol MgO x 0.752 = 0.820 mol MgO MW MgO = (24.30 + 16.00) g/mol = 40.30 g/mol MgO 0.820 mol MgO x 40.30 g MgO 1 mol MgO = 33.0 g MgO