* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download PRESENTATION NAME
Survey
Document related concepts
Abyssal plain wikipedia , lookup
Marine debris wikipedia , lookup
Marine life wikipedia , lookup
Indian Ocean wikipedia , lookup
Critical Depth wikipedia , lookup
Marine microorganism wikipedia , lookup
Arctic Ocean wikipedia , lookup
Blue carbon wikipedia , lookup
Anoxic event wikipedia , lookup
Marine biology wikipedia , lookup
Marine habitats wikipedia , lookup
Physical oceanography wikipedia , lookup
Ocean acidification wikipedia , lookup
Effects of global warming on oceans wikipedia , lookup
Marine pollution wikipedia , lookup
Ecosystem of the North Pacific Subtropical Gyre wikipedia , lookup
Transcript
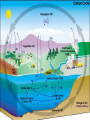
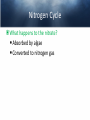
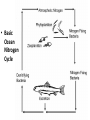
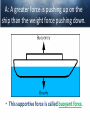
Chemistry of Seawater Aquatic Science 2011 K. Schneider The Salty Sea • The salt in the ocean exists in the form of charged particles, called ions. • Sodium and Chloride ions make up 85% of all the salt in the sea. • The rest is made up of Sulfate, magnesium, calcium, potassium & several others present in smaller quantities. Principle of Constant Proportions • Alexander Marcet (1770-1822) • The Swiss chemist and doctor carried out some of the earliest research in marine chemistry. • 1819 he discovered that all the main chemical ions (sodium, chloride, & magnesium ions) in seawater are present in exactly the same proportions throughout the world’s oceans. Sources of Salt • Some were dissolved out of rocks on land by the action of rainwater and carried to the sea in rivers. • Others enter through Hydrothermal vents, in dust blown off the land, or came from volcanic ash. Sinks • Processes that remove salts from seawater • Salt spray onto land • Precipitations of various ions onto the seafloor as mineral deposits Salinity • The amount of salt in a fixed mass of seawater. • It is determined by measuring a seawater sample’s electrical conductivity and averages about ½ oz of salt/lb of seawater. • The salinity depends on what processes or factors that are operating at that location to either add or remove water. Factors that effect Salinity • Add water, lower salinity: – High rainfall – River input – Melting of sea ice • Remove water, increase salinity: – High evaporative losses – Sea-ice formation Salinity • At depth, salinity is near constant throughout the ocean • Between the surface and deep water is in a region called Halocline, where salinity gradually increases or decreases with depth. How does the Sea smell and Taste? 1. 2. 3. 4. Have 2 members stay at their desks. Use a pencil to label the bottom of 3 cups: F, B, S Fill one cup with (F) freshwater (tap water) Fill one cup with freshwater and add a pinch of salt (B). Mix. 5. Fill one cup with freshwater and add 3 pinches of salt (S). Mix. 6. Have one student try to differentiate by tasting the water. 7. Next, have another student try to differentiate by smelling the water. Nutrients in Seawater • Nutrients – A chemical an organism needs to live and grow • Nearly constant ratio of N to P in much of the ocean 16:1 • Surface waters with little N or P, while deep waters much higher • The N:P ratio is the same in plankton as it is in water, reflecting the linkage between life and chemistry in the ocean. Nutrients in Seawater • At the base of the food chain: Phytoplankton-microscopic floating life-forms that obtain energy by photosynthesis. – Need nitrates, iron, & phosphates in order to grow – No nutrients = no growth – Too much nutrients = blooms (rapid growth phase) Phytoplankton Bloom in the Gulf of Mexico Phytoplankton blooms usually occur where cold water rushes up from the bottom of the ocean carrying nutrients to sunlit waters. In this case, the bloom may be related to recent flooding along the Mississippi River and its tributaries. Heavy rains early in December triggered floods across the southeastern United States. The draining flood water carried agricultural run-off into the Mississippi River and the Gulf of Mexico, and that may have provided the nutrients the microscopic ocean plants needed to thrive. http://eoimages.gsfc.nasa.gov/ve/19761/Mississippi_Sea_2004348.jpg Gases in Seawater • The main gases are nitrogen (N), oxygen (O2), & carbon dioxide (CO2). • The levels of O2 & CO2 vary in response to the activities of photosynthesizing organisms. Gases in Seawater • The level of O2 is generally highest near the surface, where the gas is absorbed from the air and photosynthesizers. • CO2 sinks! Concentrations are the highest at greater depths and lower at the surface. • “Soda on a warm day effect” – Higher capacity to hold a gas at a lower temperature http://eesc.columbia.edu/courses/ees/slides/climate/gas_exch.gif Qruote from: Agassiz professor of biological oceanography James McCarthy from - http://harvardmagazine.com/2002/11/the-ocean-carbon-cycle.html Carbon in the Ocean • The ocean contains the world’s largest store of CO2. • Biological and chemical processes turn some of this CO2 into the calcium carbonate shells and skeletons of organisms, other organic matter, & carbonate sediments. • However, the CO2 concentration is beginning to acidify the oceans, threatening shell and skeleton formation in marine organisms, which then threatens the food chain Ocean’s Carbon Cycle • Single-celled marine plants (phytoplankton and other marine microalgae) take in carbon dioxide (CO2) and convert it into biomass. • By converting carbon dioxide into more complex carbon compounds, the phytoplankton effectively make atmospheric carbon available to other marine organisms Ocean’s Carbon Cycle • Bacteria eat dissolved organic C compounds secreted by the phytoplanktonphytoplankton are eaten by protozoa protozoa & phytoplankton are eaten by zooplankton eaten by fish passing the carbon through the food chain and into animals like seals & polar bears • When any of these organisms die without then being consumed, or when they defecate, the carbon locked away in their bodies gradually settles to the sea floor. Ocean’s Carbon Cycle • However most of the carbon captured from the atmosphere by phytoplankton never reaches a polar bear. Some will be lost back to the water and atmosphere as the different plankton species respire. • And a vast amount will be retained in the microscopic community of phytoplankton, bacteria, and viruses living near the sea surface 10 13 11 12 Organic matter – Comes from a living Nitrogen Cycle organism, is capable of decaying, and is • Nitrogen gas (N2) made of carbon-based –compounds. Nitrous oxide (N O) 2 – Nitric oxide (NO) • “Fixed” Nitrogen Organic: •N has to be taken – Inorganic nitrogen: from the atmosphere •DNA • Nitrate (NO ) and converted intosucrose a • Nitriteplants), (NO ) •table sugar or (from usable form, either • Ammonium (NH ) C12H through lightning 22O11 or 3 2 + 4 “nitrogen fixing” bacteria. • Organic nitrogen: – Detritus and Living biomass – Dissolved organic matter (DOM) Inorganic: • Proteins/Amino Acids (ammonia) •table salt or sodium chloride, NaCl • Urea •carbon dioxide, CO2 • Nucleic Acids HOW? Ammonia HOW? HOW? Ammonium Nitrogen Fixation Nitrogen used to form ammonium N2 + 6 e- + 8H+ ---> 2 NH4+ (ammonium ion) Another way by which ammonia may be formed is by the process called nitrification. In this process nitrates and nitrites, released by decaying organic matter are converted to ammonium ions by nitrifying bacteria NO3- (nitrate ion) + 2e- + 2H+ -----------> NO2- + H2O (nitrite ion)NO2- + 6e- + 2H+ ----------> NH4+ + 2 H2O BACK http://library.kcc.hawaii.edu/external/ chemistry/everyday_nitrogen.html Nitrogen Fixation • Another way in which molecular nitrogen is modified is via the discharge of lightning. The tremendous energy released by the electrical discharges in our atmosphere breaks the rather strong bonds between nitrogen atoms, causing them to react with oxygen. lightning N2 + O2 --------------> 2 NO (nitric oxide) http://library.kcc.hawaii.edu/external/chemi stry/everyday_nitrogen.html Marine Nitrogen Cycle Courtesy of Karen Casciotti Nitrogen Cycle The role of bacteria: Convert harmful ammonia into non-toxic nutrients. ○Nitrosomonas – convert ammonia (NH4) into nitrite (NO2). ○Nitrobacteria – convert nitrite (NO2) into nitrate (NO3). ○These processes together are called nitrification. Nitrogen Cycle What happens to the nitrate? Absorbed by algae Converted to nitrogen gas Courtesy of Karen Casciotti • Basic Ocean Nitrogen Cycle Temperature • Temperature varies depending on location and depth. Temperature in the Tropics & Subtropics • In the Tropics and Subtropics, solar heating keeps the ocean surface warm throughout the year. • Below the surface, the temp declines steeply to about 4650° F at a depth of 3,300ft. – The boundary that separates the surface layers from the deep parts of the ocean is called Thermocline • 36° F on the sea floor http://www.windows2universe.org/earth/Water/tem p.html Temperature in Mid-latitudes & Polar Oceans • In mid-latitudes there is much more marked seasonal variations in surface temp. • In high latitudes and polar oceans, the water is constantly cold, sometimes below 32 °F. Density & Buoyancy • Density is the mass of a substance per unit volume (usually measured in grams per milliliters, g/ml). • Buoyancy is the upward force that a fluid exerts on an object less dense than itself. • Depends on temperature and salinity. • Decrease in temp makes seawater denser, unless the water is below 39 F (4 C), than it is a little less dense. Density • Processes that change the density of seawater cause it to either rise or sink, and drive largescale circulation in the oceans between the surface and deep water. Q: How do Ships Float? A: A greater force is pushing up on the ship than the weight force pushing down. • This supportive force is called buoyant force. Density & Buoyancy • If the buoyant force is equal to the object’s weight, it will float. • If the buoyant force is less than the object’s weight, it will sink. Archimedes’ Principle • Bouyant force was explained by Archimedes, a Greek mathematician around 3rd century B.C., and it became known as Archimedes’ Principle. • Archimedes’ Principle states that an objects weight will cause the object to sink while at the same time displacing the fluid. Archimedes’ Principle • If the weight of the water displaced becomes equal to weight of the object, it floats. • If the weight of the water displaced becomes less than the weight of the object, it sinks. • Archimedes’ Principle is important b/c: Properties of fluids ultimately determine the design of ships, airplanes, cars, and hydraulic machines. Nutrient Upwelling & Turnovers • An upwelling is an oceanographic phenomenon that involves wind-driven motion of dense, cooler, and usually nutrientrich water towards the ocean surface, replacing the warmer, usually nutrientdepleted surface water Pressure • Scientists measure pressure in units called bars. • At sea level, the atmosphere exerts a pressure of about 1 bar. • Underwater, pressure increases by 1 bar every 33ft. • Divers must breathe pressurized air or other gas mixtures. Boyle’s Law • If the temperature of a gas does not change, Its volume decreases as pressure increases and vice versa •Fish have a swim bladder, gas filled space for buoyancy. Swim bladder expands or explodes if brought to the surface to quickly. Pressure sicknesses • Nitrogen Narcosis – Nitrogen gas will dissolve better under higher pressure and therefore will be forced into body tissues – “rapture of the deep” – divers feel intoxicated by excess nitrogen – Feeling subsides as diver returns to surface Pressure sicknesses continued • Decompression sickness “The Bends” – As diver ascends to the surface, bubbles form in the blood and body tissues. – Small bubbles are of no danger (slow ascension) – Medium bubbles block smaller vessels, causing tingling and slight bruising. – Larger bubbles block blood flow to vital organs or cause nerve damage to joints(the bends). Overcoming Pressure • If diver gets decompression sickness, they must be put into a recompression chamber to relieve symptoms. • Underwater habitats – aquanauts live for several days at the depth and pressure at which they are working in specialized housing.