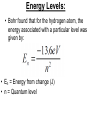* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Atomic Physics 4
Survey
Document related concepts
Molecular Hamiltonian wikipedia , lookup
Hydrogen atom wikipedia , lookup
Atomic orbital wikipedia , lookup
Electron configuration wikipedia , lookup
Arthur Compton wikipedia , lookup
Double-slit experiment wikipedia , lookup
Tight binding wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Particle in a box wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Atomic theory wikipedia , lookup
Matter wave wikipedia , lookup
Wave–particle duality wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Transcript
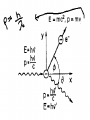
IB Physics 12 Topic 13 – Waves vs. Particles Mr. Jean The plan: • • • • • Video clip of the day Millikan’s Spotting experiment Wave Duality De Broglie Wave Length Compton Scattering De Broglie Wavelength • http://www.youtube.com/watch?v=lDYMuz o40LU Wave - Particle Duality of Light • Modern age physics accepts that light sometimes acts as a wave and at other times acts like a particle. • Both matter and electromagnetic energy exhibit some properties of waves and some properties of particles. Wave Duality: • Small Wave Length: – Behaves like a particle. • Large Wave Length: – Behaves like a wave. Compton scattering: • This was the second big experiment which proved that photons existed. • It also had some interesting results. With collisions we must consider: • Conservation of Energy: • Conservation of momentum: Compton scattering: • Major discovery supporting Quantum Theory Compton Scattering: • Compton scattering depends on how big the wave is as it interacts with the material’s surface in comparison to the Compton wave length. Compton Scattering • http://www.youtube.com/watch?v=fI2C4VlR1OM • http://www.youtube.com/watch?v=fI2C4VlR1OM &list=PLFIIOUbmbUQI6vJD0kvxqcNoRAGXZ0dx – This is a list of different videos explaining (some with examples) of the Compton scattering model. • The Problem: – Electrons moving around a nucleus in roughly circular or elliptical paths would feel a centripetal force and would therefore have centripetal acceleration. – But accelerating charges produce electric magnetic radiation, therefore these electrons should continuously emit energy, slow down, and eventually collapse into the nucleus. – Obviously, this does not happen. Bohr's Explanation: • Laws of electromagnetism do not apply at atomic level. • Within an atom energy is only absorbed or emitted when electrons change energy levels and this energy is QUANTIZED. • electrons are either in lowest energy state (ground state) or certain allowed levels (excited state). • To move from one level to another, electrons must emit or absorb a photon with a certain amount of energy (E = hf). • This absorbed or emitted photon had an energy value equal to the difference between energy levels. Energy Levels: • Bohr found that for the hydrogen atom, the energy associated with a particular level was given by: • En = Energy from change (J) • n = Quantum level • Where n is principle quantum number (energy level) and energy is negative because energy is being added to pull the electron away from the nucleus. Changing Energy Levels to Electrons: • This is a great website highlighting states of energy in matter. • http://www.kcvs.ca/martin/astro/au/unit2/6 1/chp6_1.html For the remainder of class: • Review Topics – SL Review material on Topic #4 – HL Review