* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download C. 1
History of quantum field theory wikipedia , lookup
Interpretations of quantum mechanics wikipedia , lookup
Erwin Schrödinger wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
Hidden variable theory wikipedia , lookup
Renormalization wikipedia , lookup
Elementary particle wikipedia , lookup
EPR paradox wikipedia , lookup
Dirac equation wikipedia , lookup
Copenhagen interpretation wikipedia , lookup
Identical particles wikipedia , lookup
Quantum state wikipedia , lookup
Quantum key distribution wikipedia , lookup
Density matrix wikipedia , lookup
Canonical quantization wikipedia , lookup
Renormalization group wikipedia , lookup
Schrödinger equation wikipedia , lookup
Atomic theory wikipedia , lookup
Path integral formulation wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
Coherent states wikipedia , lookup
Wave function wikipedia , lookup
Hydrogen atom wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Wheeler's delayed choice experiment wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Probability amplitude wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Particle in a box wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Double-slit experiment wikipedia , lookup
Delayed choice quantum eraser wikipedia , lookup
Matter wave wikipedia , lookup
Wave–particle duality wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
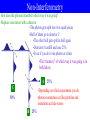
Physics 741 – Graduate Quantum Mechanics I Everyone Pick Up: •Syllabus •Two homework passes Eric Carlson “Eric” “Professor Carlson” Olin 306 Office Hours always 758-4994 (o) 407-6528 (c) [email protected] Materials •Quantum Mechanics, by Eric Carlson (free on the web) •Calculator •Pencils or pens, paper http://users.wfu.edu/ecarlson/quantum 8/26 Dr. Carlson’s Approximate Schedule 9:00 10:00 11:00 12:00 1:00 2:00 3:00 4:00 5:00 Monday Tuesday Wednesday Thursday Friday PHY 741 PHY 215 office hour office hour PHY 741 PHY 215 office hour office hour PHY 741 PHY 215 research research Free food colloquium research faculty fellow •I will try to be in my office Tues. Thurs. 12-1 •When in doubt, call/email first Reading Assignments / The Text http://users.wfu.edu/ecarlson/quantum • I use my own textbook • Downloadable for free from the web • Assignments posted on the web • Will be updated as needed • Readings every day Day Today Friday Monday ASSIGNMENTS Read Homework 1A, 1B none 1C, 1D, 1E 1.1 2A, 2B, 2C 1.2, 1.3, 1.4 Homework http://users.wfu.edu/ecarlson/quantum • • • • • Almost all the problems in the textbook About two problems due every day Homework is due at 12:00 on day Late homework penalty 20% per day Two homework passes per semester • Working with other student is allowed • Seek my help when stuck • You should understand anything you turn in Day Today Friday Monday ASSIGNMENTS Read Homework 1A, 1B none 1C, 1D, 1E 1.1 2A, 2B, 2C 1.2, 1.3, 1.4 Attendance and Tests Attendance •I do not grade on attendance •Attendance is expected •Class participation is expected •I take attendance every day Tests •Midterm will be about two hours long, approximately on Oct. 15 •Final exam will be about three hours long, 2:00 PM on Dec. 9 Grades, Pandemic Plans Percentage Breakdown: Homework 50% Midterm 20% Final 30% Grade Assigned 94% A 77% C+ 90% A73% C 87% B+ 70% C83% B <70% F 80% B- Pandemic Plans •If there is a catastrophic closing of the university, we will attempt to continue the class: •Some curving possible Emergency contacts: Web page email Cell: 336-407-6528 1. Introduction 1A. Quantum Mechanics is Weird Photons and Quantum Mechanics E hf •Light come in packets with energy •This equation is bizarre –It implies both that EM energy is in waves (f) and is particles (E) •A plane wave looks something like E r, t E0 cos kx t or E r, t E0 sin kx t •Mathematically simpler (and crucial for E r, t E0eikxit quantum) to combine them into complex waves: •Light moves at the speed of light c: ck • A formula from relativity for massless particles: E cp •Put these formulas together: p k cp E ck Uncertainty Principle for Photons •Waves are not generally localized in space p k –They have a spread in position x •You can make them somewhat localized by combining different wave numbers k –Now they have a spread in k, k •There is a precise inequality relating these two quantities xk 12 –Proved formally in a later chapter •If we multiply this equation by , we get the uncertainty principle xp 12 •In quantum mechanics, photons can’t have both a definite position and a definite momentum It’s All Done with Mirrors •Ordinary mirrors reflect all of the light that impinges on them mirror •Half-silvered mirrors have a very thin coating of metal –They reflect only half the light and transmit the other half half-mirror •What happens if we shine photons on a half silvered mirror? Photons and half-silvered mirror Let’s send photons through a half-mirror •The photon gets split into two equal pieces •Each detector sees 50% of the original photons •Even if we send photons in one at a time •Never in both detectors •If you send in a wave the other way, the same thing happens •There’s a “phase difference”, but since we square the amplitude, the probabilities are the same •50% in each detector 50% A 50% Detectors B 50% 50% Interferometry Now use two mirrors and two half-mirrors •We can reconstruct the original waves •The photon gets split into two equal pieces •The two halves of the photons are recombined by the second half-mirror •Always goes to detector A •Even one photon at a time •If you send in a wave the other way, the photon is still split in half •The “phase difference” lets it remember which way it was going A 0% •Always in detector B 100% Interferometry requires that we carefully position the mirrors 100% B 0% Non-Interferometry How does the photon remember which way it was going? •Replace one mirror with a detector •The photon gets split into two equal pieces •Half of them go to detector C •The other half gets split in half again •Detectors A and B each see 25% •Even if you do it one photon at a time •The “memory” of which way it was going is in both halves A C 25% •Depending on which experiment you do, photons sometimes act like particles and sometimes act like waves 50% B 25% Can We Have Our Cake and Eat it Too? •When you do interference, you can tell the photon went both ways •For other experiments, you can measure which way it went The plan: •Can we do both? •Do experiment in space (no friction, etc.) •Carefully measure momentum of mirror before you send one photon in •Check photon goes to detector A •Remeasure momentum and determine the path pbefore A 100% pafter B 0% p 0 k if upper path if lower path The problem •If you measure the mirror’s initial momentum accurately, you have small p, and big x •Poor positioning of mirror ruins the interference Conclusion •Because Quantum applies to photons, it must apply to mirrors as well •It seems inevitable that it applies to other things (like electrons) •We will apply it to non-relativistic systems, because these are easier to understand than relativistic – We will eventually do electromagnetism / photons, but this is harder 1B. Schrödinger’s Equation Deriving the free Schrödinger Equation in 1D •For electromagnetic field, we had waves like: E r, t E0eikxit •For non-relativistic particles, we rename the wave function: r, t Neikxit •We assume two other relationships still apply E p k •We note that: i k i x t •Multiplying by , we have: p i E i i E i p x t t x p2 •Classical relationship between the energy E and momentum p: E 2 2m p •Multiply by on the right: E 2 2m 1 i •Make the substitutions above i t 2m x Schrödinger’s Equation in 1D 2 2 p i E i i x, t x, t 2 x t t 2m x •What if we have forces? •If the forces conserve energy, they can be F x, t V x, t written as the derivative of a potential x •This contributes a new term to the energy p2 E V x, t 2m p2 E x, t x, t V x, t x, t 2m •Make the same substitutions as before 2 2 i x, t x, t V x , t x , t 2 t 2m x Schrödinger’s Equation in 3D E i t p i x •What if we are in 3D? •Momentum and potential must be generalized: •Redo the calculation as before p i F r, t V r, t p2 E V r, t 2m p2 E r, t r, t V r, t r, t 2m •Make the new substitutions: 2 i r, t 2 r, t V r, t r, t t 2m Other Things to Consider: •Particles may have spin •Some forces (such as magnetism) are not conservative forces •There may be multiple particles •The number of particles may actually be indefinite •Relativity •We will deal with all of these later 1C. The Meaning of the Wave Function Probability Density •The wave function, in essence, describes where the particle is – When it’s zero, the particle isn’t there – When it’s large, the particle more likely is there •In electromagnetism, the energy density is proportional to |E(r,t)|2 •Since the probability of finding a photon is proportional to the energy density, it makes sense to make a similar conclusion for wave functions Probability density (1D) x, t x, t * x, t x, t 2 Probability density (3D) r, t r, t * r, t r, t 2 Using Probability Density, and Normalization •The probability density must be integrated to find the probability that a particle is in a certain range x, t x, t r, t r, t P a x b x, t dx b 2 a What are the units of (x,t)? Of (r,t)? P r V r, t d r 2 3 V •The probability that a particle is somewhere must be exactly 1 1 r,t d 3r 2 1 x, t dx 2 •Don’t forget how to do 3D integrals in spherical coordinates! f r d r 3 0 2 0 0 r dr sin d d f r , , 2 2 2 Sample Problem A particle in a three-dimensional infinite square well has ground state wave function as given by N sin r R r r R , r , , 0 r R. What is the normalization constant N? What is the probability that the particle is at r < ½R? P r R N 1 2 2 1R 2 0 1 d 3r 2 N N 2 R 0 2 R 0 sin r R dr 2 4 N 2 4 N 2 1 2 R 0 1 1 sin 2 r R dr R 2 R N 2 d cos 2 0 d 2 1 N 2 R > integrate(sin(Pi*r/R)^2,r=0..R); > integrate(sin(Pi*r/R)^2,r=0..R/2); 2 r dr sin d d sin r R r 0 0 2 2 r dr sin d d sin r R r 0 0 2 4 12 R 2 4 1 sin r R dr R 2 R 0 2 R 4 2 P r 12 R 12 Some comments 2 i r, t 2 r, t V r, t r, t t 2m 1 r,t d 3r 2 •Schrödinger’s equation is first order in time •(r,t = 0) determines (r,t) at all times •Phase change in (r,t) does not affect anything – (r,t) ei (r,t) is effectively equivalent – We will treat these as different in principle but experimentally indistinguishable •Normalization condition must be satisfied at all times – Can be shown that if true at t = 0, Schrödinger’s equation makes sure it is always true – Will be proven later •We still need to discuss how measurement changes (r,t) – Chapter 4 1D. Fourier Transform of the Wave Function The Fourier Transform In 1D •The Fourier transform of a function is given by* •You can also Fourier transform in reverse •If the function is normalized, so is its Fourier transform k 2 dk x 2 dx 1 k x dx x eikx 2 dk k e ikx 2 x Neik0 x •Suppose we have a plane wave: •It’s momentum is k0 k N 2 k k0 •It’s Fourier transform is •You would expect, if the Fourier transform is concentrated near k0, then its momentum would be around k0 •This suggests the Fourier transform tells you P p k ~ k 2 something about what the momentum is *The factors of 2 differ based on convention. I like this one. The Fourier Transform and Momentum dk ikx dx ikx x k e k x e 2 2 •The wave function (x) tells you the probability of finding the particle’s position x in a certain range x2 2 P x1 x x2 x dx x1 •The Fourier Transform tells you the probability of finding the particle’s momentum p in a certain range P k1 p k2 k dk k2 2 k1 •Neither of these two representation is really more fundamental than the other •We can similarly do Fourier transforms in 3D k d 3r 2 r e 3/2 ik r r d 3k 2 ik r k e 3/2 Expectation Values •In any situation where you have a list of probabilities of a particular outcome, the expectation value is the average of what you expect to get •If there is a continuous distribution of possibilities, this becomes an integral a a P a a a a a da •For example, we can measure the average value of the position x or the position squared x2 2 2 2 2 x x x dx , x x x dx •Using the Fourier transform, we can similarly compute the momentum or its square 2 2 2 2 p k k dk , p k k dk •There are in fact ways of finding these without doing the Fourier transform (later chapter) Uncertainty •The uncertainty of a quantity is the root mean square average of how far it deviates from its average a a P a a a a P a 2 2 a a •There is an easier way to calculate this: a a 2 a 2 2 2 2 a a a P a a 2 aa a P a 2 2 a a a 2 P a 2a aP a a 2 P a a2 2a a a 2 1 a a •Uncertainty in position are given by: •These satisfy: x p 1 2 a x 2 p x 2 x dx 2 2 2 k k dk 2 2 x x dx 2 2 k k dk 2 2 Understanding from Uncertainty x p 12 •We can often gain a qualitative understanding of what is going on by considering the uncertainty principle •What stabilizes the Hydrogen atom? •It has potential and kinetic energy: 1 2 ke e 2 E p •Classically, it wants: 2m r – p = 0 to minimize the kinetic energy – r = 0 to minimize the potential energy •But this violates the uncertainty principle! •If we specify the position too well, the momentum will be large •If we specify the momentum too well, the position will become uncertain •A compromise is the best solution Sample Problem Classically, the Hydrogen atom has x p 12 energy given by •Let the uncertainty in the position be x a 1 2 ke e 2 E p •By the uncertainty principle, the momen2m r p tum must have at least uncertainty 2a •Assume the position (r) is about x from Using the uncertainty principle, estimate the ground state energy of the ideal position (r = 0), and the r a momentum (p) is about p from the hydrogen atom p the ideal momentum (p = 0) 2a 2 2 •Substitute into the energy formula ke e •The minimum is at some finite value of a: E 8ma 2 a 2 2 ke e 2 dE 2 0 3 a 4ma a da 4mke e2 •Off by factor of 4 •Substitute back into energy formula •Mostly because we ignored that 2 2 2 2 4 it was a 3D problem (factor of 3) 4mke e 2 4 mk e 2 k 2 e ee m E ke e 2 2 2 •Partly because it’s an estimate 8m