* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 1 - Physical Pharmacy Laboratory
Survey
Document related concepts
Discovery and development of cephalosporins wikipedia , lookup
Compounding wikipedia , lookup
Plateau principle wikipedia , lookup
Psychopharmacology wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription costs wikipedia , lookup
Drug design wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Neuropharmacology wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmacognosy wikipedia , lookup
Transcript
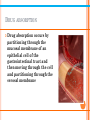
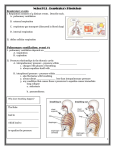
SOLUBILITY AND PARTITION PHENOMENA CONTENTS Solubility • Definitions • Factors influencing solubility • The effect of pH on solubility • The effect of pressure • Influence of solvents on solubility CONTENTS Partition Phenomena • Partition concepts • For weak electrolytes as solutes • Drug absorption • pH partition hypothesis • Site of drug partitioning DEFINITIONS Solubility is the concentration of a solute when the solvent has dissolved all the solute that it can at a given temperature • Martin’s Physical Pharmacy & Pharmaceutical Sciences 6th edition Patrick J. Sinko FACTORS INFLUENCING SOLUBILITY Dissolution of a crystal 1. A solute (drug) molecule is removed from its crystal 2. A cavity for the molecule is created in the solvent 3. The solute molecule is inserted into this cavity * Physicochemical Principles of Pharmacy 4th edition Alexander T Florence and David Attwood FACTORS INFLUENCING SOLUBILITY Temperature Solubility is an equilibrium value that is dependent on a given temperature and pressure Solubility does not depend on how fast a substance dissolves in the solvent FACTORS INFLUENCING SOLUBILITY Temperature Heat of solution (ΔHsoln) The enthalpy change associated with the dissolution of a substance in a solvent * Applied Physical Pharmacy Mansoor M.Amiji, Beverly J.Sandmann * Table. Heat of solution Solute ΔHsoln (25℃) kcal/mole HCl - 17.96 NaOH - 10.6 CH3COOH - 0.36 NaCl + 1.0 KMnO4 + 10.4 Mannitol + 5.26 KI + 4.3 FACTORS INFLUENCING SOLUBILITY Temperature S ∝exp (-ΔHsoln/RT) If ΔHsoln is negative, increasing the temperature of the solvent will decrease the solubility of the solute FACTORS INFLUENCING SOLUBILITY Chemical structure Dipole moment A measure of polarity Molecules that have a high dipole moment are more soluble in polar solvents such as water FACTORS INFLUENCING SOLUBILITY Chemical structure Dielectric properties Related to the ability to store charge How a substance interacts with solvents * Applied Physical Pharmacy Mansoor M.Amiji, Beverly J.Sandmann * Table 6-5 Dielectric Constants of Some Solvents at 25℃ Solvent Dieletric constant Water 78.5 Glycerin 40.1 Propylene glycol 32.01 Methanol 31.5 Acetone 19.1 PEG 400 12.5 Cottonseed oil 6.4 Ether 4.3 FACTORS INFLUENCING SOLUBILITY Chemical structure Hydrogen bonding The attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine Major factor on solubility in water • Amines with small alkyl groups are very soluble in water. This is because hydrogen bonding occurs between the amine and water molecules FACTORS INFLUENCING SOLUBILITY Chemical structure * Hydrogen bonding The attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine Major factor on solubility in water * Physicochemical Principles of Pharmacy 4th edition Alexander T Florence and David Attwood • The presence of hydroxyl groups or nitro groups can markedly increase the solubility. FACTORS INFLUENCING SOLUBILITY A high melting point means low water solubility * cis (z) Isomer is more soluble than trans (e) isomer * Physicochemical Principles of Pharmacy 4th edition Alexander T Florence and David Attwood # Kirk-Othmer Encyclopedia of Chemical Technology 18 OCT 2001 THE EFFECT OF PH ON SOLUBILITY Acidic drugs (HA) Total saturation solubility of drug S, the solubility of the undissociated species HA S0 S = S0 + (concentration of ionised species) * Physicochemical Principles of Pharmacy 4th edition Alexander T Florence and David Attwood THE EFFECT OF PH ON SOLUBILITY Acidic drugs (HA) The dissociation constant Ka According to previous page, [HA] = S0, [A-] = S – S0 THE EFFECT OF PH ON SOLUBILITY Acidic drugs (HA) Taking logarithms, [A-] = S – S0, S0 = [HA] <Henderson-Hasselbalch Equation> THE EFFECT OF PH ON SOLUBILITY Basic drugs (RNH2) Total saturation solubility of drug S, the solubility of the undissociated species RNH2 S0 S = S0 + (concentration of ionised species) * Physicochemical Principles of Pharmacy 4th edition Alexander T Florence and David Attwood THE EFFECT OF PH ON SOLUBILITY Basic drugs (RNH2) The dissociation constant Kb According to previous page, [RNH2] = S0, [RNH3+] = S – S0 THE EFFECT OF PH ON SOLUBILITY Basic drugs (RNH2) Taking logarithms, [RNH3+] = S – S0, S0 = [RNH2] <Henderson-Hasselbalch Equation> THE EFFECT OF PRESSURE Henry’s Law C2= σp C2= concentration of the dissolve gas (g/l) p = partial pressure (mm of the undissolvegas) σ= inverse of the Henry’s law constant, K In a dilute solution at constant temperature, the concentration of dissolved gas is proportional to the partial pressure of the gas above the solution at equilibrium INFLUENCE OF SOLVENTS ON SOLUBILITY Strong electrolytes Strong acids and bases and all salts are soluble in water The polar nature of water attracts the ions Weak electrolytes Weak acids and bases with high molecular weight are not soluble in water Cosolvents such as alcohol, propylene glycol and polyethylene glycol are required for solubility INFLUENCE OF SOLVENTS ON SOLUBILITY Cosolvents Solubility enhancements caused by cosolvent addition occur because of changes in the bulk properties of the isotropic solution PARTITIONING CONCEPTS A solute distributes itself between two immiscible solvents so that the ratio of its concentration in each solvent is equal to the ratio of its solubility in each one C0 = molar concentration in organic layer Cw = molar concentration in aqueous layer P = partition coefficient or distribution constant FOR WEAK ELECTROLYTES AS SOLUTES The partition law for weak electrolytes depends on pH Kd1 = the apparent partition coefficient and its value depends on pH FOR WEAK ELECTROLYTES AS SOLUTES Using the Ka expression, an equation can be developed that gives the hydrogen ion dependency Substitution in Kd1 gives FOR WEAK ELECTROLYTES AS SOLUTES Substitution of Kd for (HA)0/(HA)w gives This equation can be made linear FOR WEAK ELECTROLYTES AS SOLUTES The partition law for weak electrolytes depends on pH Kd1 = the apparent partition coefficient and its value depends on pH FOR WEAK ELECTROLYTES AS SOLUTES Using the Ka expression, an equation can be developed that gives the hydrogen ion dependency Substitution in Kd1 gives FOR WEAK ELECTROLYTES AS SOLUTES Substitution of Kd for [B:]0/[B:] gives This equation can be made linear DRUG ABSORPTION Drug absorption occurs by partitioning through the mucosal membrane of an epithelial cell of the gastrointestinal tract and then moving through the cell and partitioning through the serosal membrane DRUG ABSORPTION Factors influencing drug absorption Lipid/water partition coefficient (P) Water solubility Molecular weight Chemical structure DRUG ABSORPTION SMEDDS (Self-microemulsifying drug delivery system) * Lipid/water partition coefficient and water solubility are increased by using SMEDDS Drug absorption is increased and bioavailability is increased too. * European Journal of Pharmaceutics and Biopharmaceutics 76 (2010) 475 - 485 PH PARTITION HYPOTHESIS Drug absorption is a function of pH for weak electrolytes because pH changes ionization Only the uncharged molecular form of the organic weak electrolytes is absorbed → The unionized drug is provided for aqueous extracellular medium and out of the cell PH PARTITION HYPOTHESIS Using Henderson-Hasselbalch equation I = ionized drug (dissociated weak acid) U = unionized drug (associated weak acid) PH PARTITION HYPOTHESIS Calculating the % ionization of papaverine at pH = 3.9 Papaverine is a weak base, pKa = 5.9 PH PARTITION HYPOTHESIS If pH = pKa ± 2, a small change in pH will result in a large change in ionization SITE OF DRUG PARTITIONING In the human body, the ratios calculated would be for only an instant as the drug enters into tissue and is metabolized and eliminated Absorption from the stomach (pH 1 to 3) The weaker the acid (pKa > 7), the greater the percent unionized in the stomach SITE OF DRUG PARTITIONING Absorption from the intestines (pH 4 to 6) Urinary excretion (pH 5 to 7) The weaker the base (pKa < 6), the grater th percent ionization in the intestine Barbiturate poisoning may be treated by increasing the pH of plasma and urine, which clears barbiturate ion from the body Excretion of drugs in sweat (pH 5-7) Excretion of drugs in human milk (pH 6.6) Rectal administration (pH 7.8)