* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Atomic Masses
Survey
Document related concepts
Atomic nucleus wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Abundance of the chemical elements wikipedia , lookup
Inductively coupled plasma mass spectrometry wikipedia , lookup
Biochemistry wikipedia , lookup
Chemical element wikipedia , lookup
Chemical bond wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Mass spectrometry wikipedia , lookup
Allotropes of carbon wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Stoichiometry wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Isotopic labeling wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Transcript
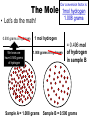
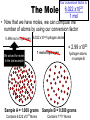
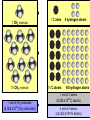
Atomic Mass and The Mole Unit: The Mole and Atomic Mass Topic: AMU’s & Atomic Mass Objectives: Day 1 of 3 • To learn how we define 1 amu (atomic mass unit) • To learn how we derive atomic mass from amu (atomic mass unit) • To learn how atomic mass is calculated using the average natural abundance of isotopes Quickwrite Answer one of the questions below 1-2 sentences: • 1 light year (the distance light travels in a year) is equal to 9.5 trillion kilometers!!!!!!!!!! Why do you think we measure distances to nearby stars in light years and not kilometers????? • Consider the reaction below, In order to make one molecule of CO2 (g), each carbon atom needs how many molecules of oxygen gas???? C(s) + O2 (g) CO2 (g) Atomic Mass • The balanced Chemical equation for the reaction of solid carbon and gaseous oxygen to form gaseous carbon dioxide is as follows: • Now suppose you have a small pile of carbon, and you want to know how many oxygen molecules are required to C(s) + O2 (g) CO2 (g) convert all this carbon into 1 Atom reacts with 1 Molecule to yield 1 Molecule carbon dioxide • The balanced equation tells us that one oxygen molecule (O2) is required for each carbon atom (C) Atomic Mass • To determine the number of oxygen molecules required, we must know how many carbon atoms are present in the pile C(s) + O2 (g) CO2 (g) of carbon 1 Atom reacts with 1 Molecule to yield 1 Molecule • But individual atoms are to small to see • We can easily count things like jelly beans and pennies, but atoms are far too small to be counted Atomic Mass • The mass of one proton in a carbon atom is 1.66 x 10-24 grams • You can’t exactly put a carbon proton on a scale and weigh it • To simplify things, and to avoid using very small complex numbers like 1.66 x 10-24 grams, scientists have defined a unit of mass called the atomic mass unit or amu for short • 1 AMU = 1.66 x 10-24 grams or 1/12 the mass of a carbon atom Atomic Mass • The mass of one carbon proton is equal to 1 AMU or 1.66 x 10-24 grams • Neutrons and protons weigh the same and electrons virtually have no mass or weight • So how many protons and neutrons does carbon have? • That’s right, 12, so carbon has 12 atomic mass units • Or, we say carbon has an average atomic mass of 12.01 AMU What is Atomic Mass Unit (amu)? 1/12 • A unit of mass equal to _____the mass of a carbon atom • 1 amu = 1/12 the mass of a ______ carbon atom or 1.66 x 10-24 grams simplify the mass • It is a way to _______ of a proton or ________ neutron Answer Bank average 1/12 simplify Carbon abundance neutron Atomic Mass • A hydrogen atom is composed of one proton or 1 amu • Remember, neutrons and protons weigh the same and electrons virtually have no mass or weight • A helium has 2 protons and 2 neutrons • What is the average atomic mass for Helium? • That’s right, 4! What is the average atomic mass for some common elements? Element Hydrogen Average Atomic Mass 1.008 Helium 4.00 Carbon 12.01 Nitrogen 14.01 Oxygen 16.00 Aluminum 26.98 Sodium 22.99 Atomic Mass • The average atomic mass for carbon is 12.01 amu • Where does the 0.01 come from? • 0.01 is the percent abundance in nature of the carbon isotopes • For example, if we weighed 12 grams of carbon, 0.01 percent is the amount of Carbon 14 and carbon 13 isotopes that exist in nature Atomic Mass • Atomic Mass is the weighted average mass of the atoms in a naturally occurring element • It is based on AMU’s and the natural abundance of an elements isotopes What is atomic mass? average mass of • The weighted _______ the atoms in a naturally occurring element • It is based on AMU’s and the natural _________ abundance of an elements isotopes Answer Bank average 1/12 simplify Carbon abundance neutron Atomic Mass • Now that we know the average mass of a carbon atom, we can count carbon atoms by weighing samples of natural carbon • For example, let’s say you want to count out 100 carbon atoms • Because 12.01 amu is the average atomic mass, Mass of 100 natural carbon atoms = 100 atoms 12.01 amu 1 carbon atom = 1,201 amu Atomic Mass • Now let’s assume that when we weigh the pile of natural carbon mentioned earlier, the result is 3.00 x 1020 amu • How many carbon atoms are present? • We know that carbon has an average atomic mass of 12.01 amu, so we can compute the carbon atoms using the following conversion factor 1 carbon atom • 1 carbon atom = 12.01 amu or 12.01 amu 3.00 x 1020 amu 1 carbon atom 12.01 amu = 2.50 x 1019 carbon atoms Practice: • Calculate the mass or weight in amu of a sample of aluminum that contains 75 atoms 75 aluminum atoms 26.98 amu = 2,024 amu 1 aluminum atom Practice: • How many aluminum atoms are present in a sample of aluminum that has a mass of 2,024 amu? 2,024 amu 1 aluminum atom = 75 atoms 26.98 amu Summarize: Why do scientist’s use AMU’s and light years??? What is the mass, in AMU of one nitrogen atom??? 1 amu = 1/12 the mass of a _____ nucleus or _________ grams ______ _____ is the weighted average mass of the atoms in a naturally occurring element It is based on AMU’s and the natural ______ of elemental isotopes Unit: The Mole and Atomic Mass Topic: The Mole Objectives: Day 2 of 3 • To understand the quantity of a mole • To understand the huge quantity of Avogadro’s number • To understand the mole mass relationship Quickwrite Answer one of the questions below 1-2 sentences: • Your pencil uses graphite (pure carbon) to write with; how many atoms do you think are in 12 grams of graphite or carbon???????? • How many items make up a dozen????? How many items make up a half dozen???? How many items are in two dozen?? The Mole • In the previous section we used atomic mass units for mass, but these are extremely small units • In a laboratory, the gram is the preferred and more convenient unit for mass • Remember our sample of carbon? • It weighs 12.01 gram • But how many atoms are in a sample of carbon that weighs 12.01 grams? The Mole • • • • Lets look at the periodic table What is the atomic mass of carbon? That’s right, 12.01 A sample of carbon with a mass of 12.01 has 6.022 x1023 atoms! • The number of atoms present in 12.01 grams of carbon is called the mole 6.022 x1023 Average Atomic Mass in grams 1.008 6.022 x1023 4.00 6.022 x1023 12.01 6.022 x1023 14.01 6.022 x1023 16.00 Aluminum 6.022 x1023 26.98 6.022 x1023 22.99 How many atoms does How many Element How many 1.008 grams ofis, How many atoms does The point atoms does hydrogen contain? atoms does 14.01 grams of a sample 4.00 grams of 12.01 grams of nitrogen contain? Of any element helium contain? Hydrogen carbon contain? that weighs A number of Helium 23 6.022 x10 grams equal Carbon To the average 23 6.022 x10 atomic mass of Nitrogen 23 6.022 x10 that element contains Oxygen 23 6.022x10 x10 23 atoms! 6.022 Sodium Number of Atoms Present What is a Mole? • The amount _____ of a substance 6.022 x 1023 that contains __________ particles of a substance • It is also the number equal to the amount of carbon atoms in 12.01 _____ grams of carbon Answer Bank 12.01 Atoms 24 Amount 6.022 x 1023(2) The Mole • As it turns out, one mole of anything contains 6.022 x1023 units of that substance • Just as a dozen eggs is 12 eggs, a mole of eggs is 6.022 x1023 eggs • The mole is an incredibly large number to imagine 602,000,000,000,000,000,000,000!!!!!!!!!!!!!!!!! • We use scientific notation to simply this number • We call this unbelievably large number Avogadro’s number The Mole • If I have a dozen eggs how many eggs do I have? • If I have 2 dozen eggs, how many eggs do I have? • If I have a mole of eggs, how many eggs do I have? • If I have a 2 mole of eggs, how many eggs do I have? (2) x (6.022 x 1023) What is Avogadro’s Number? atoms in 1 mole Answer Bank • The amount of _____ 12.01 of a substance which is Atoms 24 ________ 6.022 x 1023 Amount • Just as two dozen is (2) x (12), 6.022 x 1023(2) or ____eggs, a mole of atoms 24 is equal to (2) x (6.022 x 1023 ) atoms The Mole • Consider the following sample of hydrogen atoms below (symbolized by red dots) which contains one mole (6.022 x 1023) of hydrogen atoms • Now consider another sample in which the number of hydrogen atoms is unknown Sample A = 1.008 grams Sample B = 0.500 grams The Mole • We know sample A has 6.022 x1023 hydrogen atoms • But how many atoms are in sample B? • We know the mass is 0.500 grams Sample A = 1.008 grams Sample B = 0.500 grams The Mole • Let’s consider what we know • We know that 1 mol of hydrogen atoms has a mass of 1.008 grams • Sample B has a mass of 0.500 grams which is exactly half the mass of a mole of hydrogen atoms Sample A = 1.008 grams Sample B = 0.500 grams The Mole • Let’s consider what we know • We know that 1 mol of hydrogen atoms has a mass of 1.008 grams • Sample B has a mass of 0.500 grams which is exactly half the mass of a mole of hydrogen atoms Sample A = 1.008 grams Sample B = 0.500 grams The Mole • Let’s do the math! 0.500 grams of hydrogen We know we have 0.500 grams of hydrogen Our conversion factor is: 1mol hydrogen 1.008 grams 1 mol hydrogen 1.008 grams of hydrogen Sample A = 1.008 grams = 0.496 mol of hydrogen in sample B Sample B = 0.500 grams Our conversion factor is: 23 6.022 x10 The Mole 1 mol • Now that we have moles, we can compute the number of atoms by using our conversion factor 0.496 mol of hydrogen 6.022 x1023 hydrogen atoms = 2.99 x1023 We solved for moles In the last example 1 mol of hydrogen hydrogen atoms in sample B Sample A = 1.008 grams Sample B = 0.500 grams Contains 6.022 x1023 Atoms Contains ???? Atoms Practice: • Your chicken laid 562 eggs. How many dozen eggs do you have? 562 eggs 1 dozen = 46 dozen 12 eggs Practice: • How many moles are in a 42 gram sample of aluminum? grams 42 grams Al moles 1 mol aluminum 26.98 grams Al = 1.56 mol of Aluminum Practice: • How many atoms are in 1.56 mol of aluminum? moles 1.56 mol Al number of atoms 6.022 x1023 Al atoms 1 mol Al = 9.39 x1023 Aluminum Atoms Practice: • Calculate the mass of 1.56 mol of Aluminum: moles 1.56 mol Al grams 26.98 grams Al 1 mol Al = 42 grams Aluminum Practice: • How many atoms are in a 18 gram sample of carbon? grams moles 18 grams C 1 mol C 12.01 grams C number of atoms 6.022 x1023 C atoms 1 mol C = 9.07 x1023 Carbon Atoms Summarize: • If I have a _____of something I have_______ particles • Avogadro number is ___________ • A mole of carbon atoms weighs(mass) ______grams and contains ______ atoms • 2 moles of carbons atoms weighs _____ grams • Review: An ______ is atom with a different amount of neutron than protons Unit: The Mole and Atomic Mass Topic: Molar Mass/Molecular Weight Objectives: Day 3 of 3 • To learn how to calculate Molar Mass • To learn how to convert between moles and grams • To learn how to calculate % composition Quickwrite Answer one of the questions below 1-2 sentences: • Let’s say you want to find the weight of your dog, which is too big to stand on your bathroom scale; how could you find his weight??? • Together, you and your dog weigh 100 kilograms, you know that you weigh 75 kilograms, what percent by weight does your dog weigh???? Molar Mass/Molecular Weight • A chemical compound such as methane (CH4) is a collection of atoms • Methane contains 1 carbon atom and 4 hydrogen atoms • But how do we calculate the mass of one mol of methane? • In other words, what is the mass of 6.022 x1023 C methane molecules? Molar Mass/Molecular Weight • Because each methane molecule (CH4) contains one carbon atom and 4 hydrogen atoms, 1 mol (CH4) molecules consists of 1 mol carbon atoms and 4 mol of hydrogen atoms • So the mass of 1 mol of (CH4) is equal to: Mass of 1 mol of carbon (C) = 1 x 12.01g = 12.01 g Mass of 4 mol of hydrogen (H) = 4 x 1.008 = 4.032g _______ Mass of 1 mol of (CH4) = 16.04 g 1 CH4 molecule 10 CH4 molecule 1 C atom 10 C atoms 4 hydrogen atoms 40 hydrogen atoms 1 mol of C atoms 1 mol of CH4 molecules (6.022 x1023 CH4 molecules) (6.022 x1023 C atoms) 4 mol of H atoms 4 (6.022 x1023 H atoms) What is molar mass or molecular weight? • It is the mass in grams of one _____ mole of a substance or compound • It is found by _______ adding the atomic ________that make up a compound masses Answer Bank Total Percent Element adding mole masses or molecule • Ex: methane Mass of 1 mol of carbon (C) = 1 x 12.01g = 12.01 g Mass of 4 mol of hydrogen (H) = 4 x 1.008 _______ = 4.032g Mass of 1 mol of (CH4) = 16.04 g Practice: • Calculate the molar mass of sulfur dioxide: Mass of 1 mol of sulfur (S) = 1 x 32.07 g = 32.07 g Mass of 2 mol of oxygen (O) = 2 x 16.00 g =_______ 32.00 g Mass of 1 mol of (SO2) = 64.07 g Practice: • A sample of calcium carbonate (chalk) contains 4.86 mol. What is the mass in grams of this sample: First calculate the molar mass of CaCO3: Mass of 1 mol of Calcium (Ca)=1 x 40.08 g = 40.08 g Mass of 1 mol of Carbon (C) =1x 12.01 g = 12.01 g Mass of 3 mol of Oxygen (O) =3 x 16.00 g = _______ 48.00 g Mass of 1 mol of (CaCO3): = 100.09 g 4.86 mol CaCO3 100.09 grams CaCO3 1 mol CaCO3 = 486 grams CaCO3 Practice: • A sample of water weighs 20.56 grams. How many water molecules are in a sample of water that weighs 20.56 grams? 20.56 gms H2O 1 mol of H2O 18.016 gms H2O 6.022 x1023 H2O molecules 1 mol H2O =6.87 x 1023 molecules Percent Composition • Chemists often need to know a chemical’s composition in terms of the masses of it’s elements • We can obtain this information from the formula of the compound by comparing the mass of each element present in 1 mol of the compound to the total mass of 1 mol of the compound Percent Composition • The mass fraction for each element is calculated as follows: Mass Mass of the element present in 1 mol of compound for a given = Mass of 1 mol of compound element • For example, lets consider ethanol (C2H6O): • We calculate the mass of each element present and the molar mass of ethanol as follows: Mass of C = 2 mol C 12.01 grams C = 1 mol C Mass of H = 6 mol H 1.008 grams H = 6.048 g H 16.00 grams O 1 mol O x100 = 13.13% H 46.07 g C2H6O 1 mol H Mass of O = 1 mol O 24.02 g C x100 = 52.14 % C 46.07 g C2H6O = 16.00 g O x 100 = 34.73% O 46.07 g C2H6O Mass of 1 mol of C2H6O ethanol = 46.07 g = molar mass • The mass percent of carbon in ethanol can be computed by comparing the mass of carbon in 1 mol of ethanol with the total mass of 1 mol of ethanol and multiplying the result by 100% Mass % of C = Mass of C in 1 mol C2H6O = 24.02 g x 100% = 52.14% Mass of 1 mol C2H6O 46.07 g Mass % of H = Mass of H in 1 mol C2H6O 6.048 g x 100% = 13.13% Mass of 1 mol C2H6O = 46.07 g Mass of O in 1 mol C2H6O = 16.00 g x 100% = 34.73% Mass % of O = Mass of 1 mol C2H6O 46.07 g Percent Composition • To review, by weight, ethanol contains 52.14% carbon, 13.13% hydrogen, and 34.73% Oxygen • Or, 52.14% +13.13% + 34.73% = 100% What is Mass Percent? percent • The ________by mass of an Answer Bank Total element in a compound or ________ Percent molecule Element adding mole • Mass % is calculated by masses comparing the mass of a single total mass element to the _____ (molar mass) of the compound Practice: • Find the weight percent of oxygen in water H2O: Practice: • First calculate the molar mass of H2O: Mass of 2 mol of hydrogen (H)= 2 x 1.008 g = 2.016 g Mass of 1 mol of oxygen (O) = 1 x 16.00 g = _______ 16.00 g Mass of 1 mol of (H2O): = 18.016 g • A sample of water weighs 20.56 grams. How many moles of water are in 20.56 grams of water? 20.56 grams H2O 1 mol of H2O 18.016 grams H2O = 1.14 mol H2O Summarize: • _____ _____is the mass in grams of one mole of a substance • To calculate Molar mass, you would add up the ____ ______for each element that make up a molecule • The percent by mass of an element in a compound is called it’s ______ ______ • Calculate the percentage of nitrogen in nitrogen dioxide NO2