* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download CH4 PT1 Arrangement of Electrons
Elementary particle wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Casimir effect wikipedia , lookup
Bremsstrahlung wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Particle in a box wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Double-slit experiment wikipedia , lookup
X-ray fluorescence wikipedia , lookup
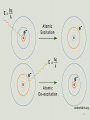
Electron scattering wikipedia , lookup
Hydrogen atom wikipedia , lookup
Tight binding wikipedia , lookup
Atomic orbital wikipedia , lookup
Electron configuration wikipedia , lookup
Matter wave wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Arrangement of Electrons in Atoms Part One 1 Section 1 Development of a New Atomic Model • Explain the mathematical relationship amount the speed, wavelength, and frequency of electromagnetic radiation. • Discuss the dual wave-particle nature of light. • Discuss the significance of the photoelecvtric effect and the line-emission spectrum of H to the development of the atomic model. • Describe the Bohr model of the H atom. 2 Section 2 Quantum Model • Discuss De Broglie’s role in the development of the quantum model of the atom. • Compare and contrast the Bohr model and the quantum model of the atom. • Explain how the Heisenberg uncertainty principle and the Schrodinger wave equation led to the idea of atomic orbitals. 3 • Electromagnetic Radiation (EMR) or light is a form of energy that moves as a wave through space. • Electromagnetic Spectrum is made up of many kinds of EMR: visible, X rays, ultraviolet, infrared, microwaves, and radiowaves. Section 3 – Electron Configuration • Aufbrau principle, Hund’s rule, Pauli exclsuion principle • Notation types (orbital, e-configuration, noble-gas) 4 Electromagnetic (E-M) Waves (LIGHT!) Do not require a medium through which to travel Light travels at 3.0 x 108 m/s in a vacuum or air Its wavelength and frequency varies according to the type of E-M wave 5 Higher frequency Greater energy More penetration What type has a. Greatest frequency? b. Less frequency than infrared light? 6 c = ln • c , the speed of light which is 3x108 m/s in a vacuum or air. Units: m/s • l, wavelength or distance between corresponding points on adjacent waves – Units: m or nm • n, frequency or number of waves passing a point in a given amount of time. Units: Hertz, Hz or 1/s or s-1 7 Light Problems: What is the frequency of light whose wavelength is 600 nm? nm means 10-9 m c = ln --> n = c/l n = c/l = 3x108 m = 5 x 1014 s-1 600x10-9 m s 8 Photoelectric Effect • This is the emission of electrons from a metal when electromagnetic radiation shines on the metal. P. E. shows that energy is emitted in small, specific packets called quanta. A quantum of energy is the minimum quantity of energy that can be lost or gained by an atom. The photoelectric effect showed that light behaves as particles, too! 9 E=hn * E, energy of a quantum of radiation in joules, J h, Planck’s constant is 6.626 x 10-34 Js n, frequency in s-1 Problem: What is the frequency of a photon whose energy is 3.4 x 10-19 J? n = E/h = 3.4 x 10-19 J / 6.626 x 10 -34 Js n = 5.1 x 1014 s-1 *Wavelength-frequency relationship was proposed by Planck in 1900. 10 • Einstein explained the photoelectric effect was due to metal absorbing energy in discrete amounts of photons. • Ground state – lowest energy state of an atom • Excited state – state where an atom has a higher potential energy than ground state. 11 webexhibits.org 12 Tutorvista.com 13 Bohr Model • 1913 – Niels Bohr proposed a hydrogen atom model where electrons circle the nucleus only in allowed paths or orbits with a definite amount of energy. • If an electron absorbs energy, it can go to a higher level. • If in a higher energy level, an electron can emit a certain amount of energy to move to a lower level. 14 • chemweb.ucc.ie 15 Quantum Model of the Atom • Questions were unanswered regarding how electrons could be particles yet they gave off waves of light. • De Broglie suggested that electrons could be considered waves confined to space around a nucleus only at specific frequencies. • Diffraction experiments proved that electron beams can interfere with each other and produce areas of low energy and high energy areas as a result of interference. 16 • Heisenberg Uncertainty Principle (1927) – it is impossible to determine simultaneously both the position and velocity of an electron or any other particle. • Schrodinger Wave Equation (1926) - developed an equation that treated electrons in atoms as waves. • Heisenberg and Schrodinger laid the foundation for mathematical descriptions of wave properties of very small particles such as electrons – the probable location of electrons around the nucleus. • AKA: Quantum Theory and Quantum Numbers. 17 • End of Section 1 and part of Section 2 of Chapter 4 • Arrangement of Electrons in Atoms 18 19 20