* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Hybridisation
Survey
Document related concepts
Homoaromaticity wikipedia , lookup
State of matter wikipedia , lookup
Coupled cluster wikipedia , lookup
Heat transfer physics wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Aromaticity wikipedia , lookup
Hartree–Fock method wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Chemical bond wikipedia , lookup
Atomic orbital wikipedia , lookup
Transcript
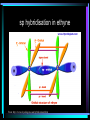
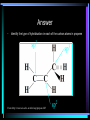
HL Bonding Hybridisation • Hybridization is a model which is used to explain the behavior of atomic orbitals during the formation of covalent bonds. • When an atom forms a covalent bond with another atom, the orbitals of the atom become rearranged. • This rearrangement results in the "mixing" of orbitals. From: http://www.bookrags.com/Orbital_hybridisation Hybridisation • When atoms join together to form molecules, their atomic orbitals interact with each other to form hybrid orbitals Hybridisation • When atoms join together to form molecules, their atomic orbitals interact with each other to form hybrid orbitals • This process is called hybridisation Hybridisation • When atoms join together to form molecules, their atomic orbitals interact with each other to form hybrid orbitals • This process is called hybridisation • The orbitals formed in this process are of the same energy Hybridisation • When atoms join together to form molecules, their atomic orbitals interact with each other to form hybrid orbitals • This process is called hybridisation • The orbitals formed in this process are of the same energy • The orbitals are symmetrically arranged Hybridisation • When atoms join together to form molecules, their atomic orbitals interact with each other to form hybrid orbitals • This process is called hybridisation • The orbitals formed in this process are of the same energy • The orbitals are symmetrically arranged • 3 types - sp3, sp2 and sp Hybridisation • When atoms join together to form molecules, their atomic orbitals interact with each other to form hybrid orbitals • This process is called hybridisation • The orbitals formed in this process are of the same energy • The orbitals are symmetrically arranged • 3 types - sp3, sp2 and sp • Hybridisation doesn’t just occur in carbon sp3 hybridisation • E.g. methane, ammonia, water sp3 hybridisation • E.g. methane, ammonia, water • Methane contains 4 equal C - H bonds, therefore the outer shell electrons (2s2 2p2) have merged to form 4 hybrid sp3 orbitals of equal energy sp3 hybridisation • E.g. methane, ammonia, water • Methane contains 4 equal C - H bonds, therefore the outer shell electrons (2s2 2p2) have merged to form 4 hybrid sp3 orbitals of equal energy • One electron is in each of the hybrid orbitals and can form a sigma bond with a hydrogen atom sp3 hybridisation in methane From http://ibchem.com/IB/ibnotes/full/bon_htm/14.2.htm sp3 hybridisation • The four hybrid orbitals arrange themselves to be as far apart as possible because of the repulsion between the electrons • This produces the tetrahedral shape of methane and a bond angle of 109.5º sp3 hybridisation in methane From http://www.mhhe.com/physsci/chemistry/carey5e/Ch02/ch4hybrid2.gif sp3 hybridisation in water • A similar thing occurs in water - 4 sp3 hybridised orbitals are formed around the oxygen and spread out in a tetrahedral shape sp3 hybridisation in water • A similar thing occurs in water - 4 sp3 hybridised orbitals are formed around the oxygen and spread out in a tetrahedral shape • Two of these orbitals contain lone/nonbonded pairs of electrons, and the other two form sigma bonds with the hydrogen atoms sp3 hybridisation in water • A similar thing occurs in water - 4 sp3 hybridised orbitals are formed around the oxygen and spread out in a tetrahedral shape • Two of these orbitals contain lone/nonbonded pairs of electrons, and the other two form sigma bonds with the hydrogen atoms • As the non-bonded pairs are closer to the centre of the molecule, they force the two OH bonds slightly closer together forming a bond angle of 105 º sp3 hybridisation in water From: http://www.rjc.edu.sg/subjects/chemistry/resources/simulations-hybridisation/lecture28.html sp3 hybridisation in water From: http://www.rjc.edu.sg/subjects/chemistry/resources/simulations-hybridisation/lecture28.html sp2 hybridisation • E.g. ethene - C2H4, BF3 sp2 hybridisation • E.g. ethene - C2H4, BF3 • In ethene, one 2p orbital from each carbon atom forms a pi bond - this is not involved in hybridisation sp2 hybridisation • E.g. ethene - C2H4, BF3 • In ethene, one 2p orbital from each carbon atom forms a pi bond - this is not involved in hybridisation • The remaining 2s orbital and two 2p orbitals hybridise to form three sp2 orbitals, which form sigma bonds - two with hydrogen atoms and one between the C atoms sp2 hybridisation in ethene From: http://www.rsu.ac.th/science/chem/yupa/Arjarnyupa/1historyorganic/sp2.htm Hybridisation in BF3 From: http://www.rjc.edu.sg/subjects/chemistry/resources/simulations-hybridisation/lecture28.html sp hybridisation • E.g. ethyne (C2H2), BeF2 sp hybridisation • E.g. ethyne (C2H2), BeF2 • The 2s orbital hybridises with just one of the 2 p orbitals sp hybridisation • E.g. ethyne (C2H2), BeF2 • The 2s orbital hybridises with just one of the 2 p orbitals • In BeF2 this is because there are only 2 electrons in the 2nd shell (1s2 2s2) sp hybridisation • E.g. ethyne (C2H2), BeF2 • The 2s orbital hybridises with just one of the 2 p orbitals • In BeF2 this is because there are only 2 electrons in the 2nd shell (1s2 2s2) • In ethyne this is because two pi bonds are formed between the carbons leaving only 2 electrons on each carbon to form sigma bonds - one with a H atom and one with the other C atom sp hybridisation in ethyne From: http://www.citycollegiate.com/hybridization2.htm sp hybridisation in BeF2 From: http://www.rjc.edu.sg/subjects/chemistry/resources/simulations-hybridisation/lecture28.html Question • Identify the type of hybridisation in each of the carbon atoms in propene From: http://www.neiu.edu/~ncaftori/eng/propene.GIF Answer • Identify the type of hybridisation in each of the carbon atoms in propene sp2 From: http://www.neiu.edu/~ncaftori/eng/propene.GIF sp3 sp2