* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Subatomic Heavyweights
Survey
Document related concepts
Transcript
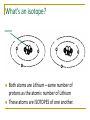
Subatomic Heavyweights Opening Questions neutron 1. What is different about the two atoms above? 2. What is the atomic number of each atom above? 3. What is the mass number of each atom? 4. Do you think they are both lithium atoms? Why? What’s an isotope? neutron Both atoms are Lithium—same number of protons as the atomic number of Lithium These atoms are ISOTOPES of one another. What’s an isotope? Isotopes: Atoms of the same element that have different numbers of neutrons. Because of the different numbers of neutrons, the atoms have different masses. neutron Lithium-6 Lithium-7 Subatomic Heavyweights Summary Atoms of the same element will ALWAYS have the same number of protons • Atomic weight: the weighted average atomic mass of the naturally occurring isotopes (the # on the periodic table) • • To find the most common isotope: round the atomic weight to the nearest whole number Subatomic Heavyweights Summary Isotope notation: Mass number Atomic number The above can also be written as: cobalt-59 Wrap-Up How do potassium-39, potassium-40, and potassium-41 differ from each other? How many electrons, protons, and neutrons are contained in each of the following atoms? 1. 2. a. b. Fluorine-23 Molybdenum-96 c. 3. What is the atomic weight of phosphorus? What is its atomic number? Predict which isotope you would find in greatest abundance for phosphorus.