* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download bixbycenter.ucsf.edu
Behçet's disease wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Common cold wikipedia , lookup
Sociality and disease transmission wikipedia , lookup
Transmission (medicine) wikipedia , lookup
Germ theory of disease wikipedia , lookup
Sarcocystis wikipedia , lookup
Marburg virus disease wikipedia , lookup
Childhood immunizations in the United States wikipedia , lookup
Globalization and disease wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Sjögren syndrome wikipedia , lookup
Hepatitis C wikipedia , lookup
Multiple sclerosis research wikipedia , lookup
Schistosomiasis wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Coccidioidomycosis wikipedia , lookup
Neonatal infection wikipedia , lookup
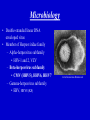
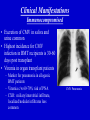
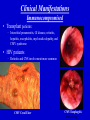
Cytomegalovirus Reproductive Infectious Disease Seminars February 15, 2005 Natali Aziz, MD, MS Reproductive Infectious Disease and Maternal-Fetal Medicine Fellow Department of Obstetrics, Gynecology and Reproductive Sciences University of California, San Francisco Overview • • • • • • Microbiology Pathogenesis Virulence Epidemiology Congenital CMV Clinical Manifestations – Immunocompetent Hosts – Immunocompromised Hosts – Congenital/Perinatal Disease • Diagnosis • Treatment – Immunocompromised • Prophylaxis – Immunocompromised • Vaccines Microbiology • Double-stranded linear DNA enveloped virus • Member of Herpesviridae family – Alpha-herpesvirus subfamily • HSV-1 and 2, VZV – Beta-herpesvirus subfamily • CMV (HHV5), HHV6, HHV7 – Gamma-herpesvirus subfamily • EBV, HHV8 (KS) www.biosciences.bham.ac.uk Microbiology • CMV – Icosahedral nucleocapsid containing dS DNA viral structure – 162 hexagonal protein capsomeres – Additional layer of surrounding protein (tegument) – Outer membrane envelope with glycoprotein complexes www.biografix.de Microbiology • Largest member of herpesviridae family – 230-240 kilobase pairs • Large cytomegalic cells with enlarged nuclei • Class E genome • Unique and inverted repeats that include the existence of 4 genome isomers caused by inversion of L-S genome components (class E). – Violaceous intranuclear inclusions surrounded by a clear halo – Basophilic stippling may be present in the cytoplasm • Replication cycle – Immediate early: 4 h – Early: 4-24 h – Late: 24 h www.som.tulane.edu Pathogenesis • Lytic virus with cytopathic effect • Initial infection – Epithelial cells of the salivary gland persistent infection and viral shedding – Genitourinary system • Proximal tubules near cortical areas – Ultimately can be found in several tissues (salivary gland, lung, liver, kidney, intestine, adrenal gland and CNS) • Other pathogenic mechanisms – Granulomatous reaction, particularly in liver – Immune complex formation – Vasculitis • Establishes a latent host infection – May reactivate during a period of immunosuppression secondary to drugs or concurrent infection (eg. HIV) Pathogenesis • • • • • Incubation period: 28-60 days Primary infection symptoms: 9-60 days Viremia: 2-3 weeks IgM response: 30-60 days Peak viral titers: 4-7 weeks post infection Virulence • US2, US11, US3, US6 – Gene products interfere with MHC class I function and antigen processing • UL33, US27, US28 – Subvert normal inflammatory process – Promote tissue dissemination of virus • MHC class I homologue – Evades host defense • UL144 – Encodes TNF homolog and may thereby escape immune clearance • Antivirals interfere with early gene products – Ganciclovir: targets UL54 and is phosporylated by UL97 protein • Genetic differences among viruses – Multiple strains of CMV – Differences in genotypes may be associated with differences in virulence – Dual infection with different strains possible Schleiss and McVoy, 20004 Immunology Humeral Immunity • Primary infection • CMV IgM antibodies may be found as early as 4-7 weeks Cell-Mediated Immunity • Most important factor controlling infection – CD4 and CD8 – Persist as long as 16-20 weeks after initial infection • Majority of neutralizing Ab directed against envelope glycoprotein gB and gH – >50% of neutralizing activity in convalescent serum attributable to glycoprotein gB • Virion tegument proteins pp150, pp28, and pp65 evoke strong and durable antibody responses Glycoprotein B (gpUL55) mediated morphogenesis of infectious CMV particles www.biografix.de Epidemiology • 50-85% prevalence in US by 40 years of age • As high as 100% prevalence in some populations • CMV prevalence increases with age • Risk factors – Work at day care/contact with children – Blood transfusion – Multiple sexual partners – Unprotected intercourse – Parity – Abnormal cervical cytology – Lower SES/underdeveloped nations – Born outside US – First pregnancy before 15 years of age – Infection with STI • Transmission: transplanted organ, breast milk, urine, saliva, tears, stool, sexual contact, blood, transplacental • Seldom associated with mortality in immunocompetent hosts (<1%) • Significant morbidity and mortality in immunocompromised (solid and BM transplant, AIDS, etc.) – Despite antiviral therapy, allogenic BMT patients will have 15-75% interstitial PNA mortality rate www.cdc.gov Congenital CMV and Pregnancy • Most common congenitally acquired infection – Occurs 0.2-2% of all neonates – Approximately 40, 000 infants infected annually in US • Leading cause of congenital hearing loss in US • Prevalence in pregnancy – Seropositive: 50-80% – Primary infection: 0.7-4% – Recurrent infection: 13.5% • Cervical excretion of CMV common during pregnancy – Not indication for c-section • CMV in breast milk – CMV not contraindication to breast feeding • Vertical transmission – Transplacental infection: symptomatic – Exposure to genital tract secretions: usually asymptomatic – Breast feeding : usually asymptomatic • Worse disease in premature infants Congenital CMV and Pregnancy • Vertical transmission greatest during 3rd trimester • More serious fetal sequelae when infection in 1st trimester • Vertical transmission – Primary maternal infection: 30-40% – Recurrent maternal infection: 0.15-2% • Symptomatic congenital CMV disease less likely in women with pre-existing immune response – Congenital hearing loss most severe sequelae • Most infants with congenital CMV are asymptomatic at birth • 10% of infants infected in-utero will develop CMV signs and symptoms at birth – Poor prognosis – 30% of severely infected die – 80% of survivors severe neurologic sequelae • 85-90% of infected infants are asymptomatic at birth – 10-15% will develop long-term neurologic sequelae, hearing loss Congenital CMV and Pregnancy www.aafp.org Congenital CMV and Pregnancy Congenital CMV and HIV • Congenital CMV is NOT more common in infants of HIVinfected mothers (Kovacs 1999, Mussi-Pinhata 1998) • Perinatal acquisition of CMV higher among HIV-infected babies (Kovacs 1999) – Dual infection associated with higher HIV progression (RR 2.59) and CNS disease • CMV and HIV potentiate each other in vitro (Skolnik 1988) Clinical Manifestations • Immunocompetent host disease • Immunocompromised host disease lbmi.org/pathologyimages • Congenital/Perinatal disease Clinical Manifestations Immunocompetent www.crprc.ucdavis.edu • Usually asymptomatic or mild flu-like symptoms • Mononucleosis syndrome – Fever/chills, malaise, myalgia – Mild hepatitis, leukocytosis, atypical lymphocytes in blood x 6 weeks – Less hepatomegaly, splenomegaly, pharyngitis than EBV – Older patients, longer fever duration, less cervical LAN – Negative Monospot or heterophile-agglutinin tests • Meningoencephalitis, pericarditis, myocarditis, thrombocytopenia, hemolytic anemia, maculopapular rash, GI ulcers, pneumonia less common • Reactivation possible – Viremia – Positive IgM in presence of IgG – In setting of concurrent infections or stress Clinical Manifestations Immunocompromised • Significant disease in the immunocompromised host – Pneumonia – Hepatitis – Encephalitis – Colitis/GI ulcerations – Uveitis – Retinitis – Neuropathy – CMV syndrome Normal Retina CMV Retinitis www.5mcc.com Clinical Manifestations Immunocompromised • Excretion of CMV in saliva and urine common • Highest incidence for CMV infection in BMT recipients is 30-60 days post transplant • Viremia in organ transplant patients – Marker for pneumonia in allogenic BMT patients – Viremia c/w 60-70% risk of PNA – CXR: miliary/interstitial infiltrate, localized/nodular infiltrates less common CMV Pneumonia Clinical Manifestations Immunocompromised • Transplant patients: – Interstitial pneumonitis, GI disease, retinitis, hepatitis, encephalitis, myeloradiculopathy, and CMV syndrome • HIV patients – Retinitis and CNS involvement more common CMV Cecal Ulcer CMV Esophagitis www.vh.org/ Clinical Manifestations Congenital/Perinatal www.med.nagoya-u.ac • • • • • • • • • Clinical Findings Laboratory Findings Petechiae/Purpura(71-76%) • Jaundice (67%) • Hepatosplenomegaly (60%) • Microcephaly (53%) • IUGR (50%) Retinitis Cerebral calcifications Hepatitis Non-immune hydrops Elevated LFT’s (83%) Hyperbilirubinemia (81%) Thrombocytpenia (77%) Elevated CSF protein (77%) Long Term Sequelae – Hearing loss – Mental retardation – Neurologic manifestations • Associated with higher IgM levels Clinical Manifestations Congenital/Perinatal Clinical Manifestations Congenital/Perinatal Ventriculomegaly and Periventricular Calcifications Diagnostic Studies • Conventional cell culture • Serology • Shell vial culture • CMV antigenemia (pp65) • Molecular methods (PCR) Cytomegalovirus-infected human diploid fibroblast cells in culture. Modified acridine orange staining [M. Battaglia, unpublished]. Diagnostic Studies • Shell vial culture – Early antigen detection with monoclonal antibodies – Viremia has been shown to be a risk factor for CMV pneumonia in patients who have received allogenic marrow transplants – Shell vial assay reduced identification time to 24-48 hours – Monitoring of the shell vial assay prior to the onset of disease • Practical method for starting early antiviral treatment – Uses permissive cell line for CMV • Centrifuged at a low speed and placed in an incubator • After 24 and 48 hours, the tissue culture medium is removed and the cells are stained using a fluoresceinlabeled anti-CMV antibody • The cells are read using a fluorescent microscope • Alternatively, the cells are stained with an antibody against CMV, followed by a fluorescein-labeled antiIg. – Not as sensitive as traditional tissue culture Diagnostic Studies • CMV antigenemia (pp65) forums.gardenweb.com – Antigenemia: relatively new test developed in the late 1980s – Recognition of CMV early antigen by a mixture of 2 mouse monoclonal antibodies, C-10 and C-1 – The detector system is fluorescein-labeled anti-mouse Ig – Cells are counted using a fluorescent microscope – Any positive cell confirms the diagnosis of CMV viremia – The literature has suggested that a higher cell count corresponds to risk of disease. – Antigenemia has been used to predict CMV pneumonia in patients who have received transplants – A positive antigenemia test result can be used as a trigger to institute ganciclovir therapy when a preemptive strategy is used for the prevention of CMV disease in transplant patients – Available in 24 hours Diagnostic Studies • Molecular methods (PCR) – Qualitative polymerase chain reaction • PCR has been used to detect CMV in blood and tissue samples • The PCR test depends on the multiplication of primers specific for a portion of a CMV gene – Primers usually bind to the area of virus that codes for early antigen • The test is extremely sensitive • Positive before the antigenemia test in patients with viremia who have received transplants. – The PCR test result is not usually positive in patients without CMV viremia. • Qualitative PCR has also been used to detect CMV in histological sections Diagnostic Studies • Molecular methods (PCR) – Quantitative polymerase chain reaction • Quantitative PCR has been used to detect plasma CMV • Quantitative PCR is as sensitive as qualitative PCR and provides an estimate of the number of CMV genomes present in plasma • Research – Determine if the number of CMV genomes present in the plasma correlates with risk of disease in different at-risk populations – Number of CMV genomes (ie, viral load) present would indicate whether therapy is necessary because patients below a certain cut-off would not develop CMV disease – However, the level of viremia necessary for CMV disease to occur may vary depending on host factors and the type of organ transplant, and this may need to be determined empirically – Literature from different organ transplant systems suggests that this method may be the test that discriminates between lowlevel viremia versus a higher level and CMV disease Maternal Diagnosis Congenital CMV • Serum tested 2-4 weeks apart • IgG seroconversion or fourfold increase (ie. 1:4 to 1:16) of IgG • IgM useful but not always reliable sign of primary infection – May persist for months – Appears in reinfection – False positive if RA – >30% of IgG value may suggest active infection www.dpcweb.com Fetal Diagnosis Congenital CMV • CMV detected in amniotic fluid by • Ultrasound findings culture or PCR – Abdominal and liver calcifications – Culture sensitivity: 50-69% – Lateral ventricle calcifications in – PCR sensitivity: 77-100% lateral border – Combined sensitivity: 80-100% – Hydrops – Comparable PPV and NPV – Echogenic bowel • Sensitivity of amniotic fluid testing – Ascites markedly lower if performed before 21 – Hepatosplenomegaly weeks GA – Ventriculomegaly • Severity not predicted by amniotic – ?Thickened nuchal translucency fluid assessment not associated with maternal infection (Sebire et al 1997) • Ultrasound may initially be normal • CNS involvement poorer prognosis Considerations Congenital CMV Therapies • No current therapies for maternal or fetal CMV infection • Ganciclovir crosses placenta in vitro • Reported use of ganciclovir and CMV IgG postnatally for congenital disease – Prevention of long-term neurologic sequelae not proven Screening? • Routine screening not recommended • IgM not reliable for differentiating primary infection • Maternal immunity does not eliminate fetal infection • Screening indications – Symptoms suggestive of CMV infection (mononucleosis-like syndrome or elevated LFT’s) – Exposure to CMV – Immunocompromised patients Antiviral Therapy for Congenital and Perinatal Infection • A phase II study has investigated intravenous ganciclovir at 8 and 12 mg/kg per day, divided every 12 hours and given for six weeks in each case (Whitley 1997) – Decreased viral excretion during drug administration – Viruria returned after drug cessation – 16% stable/improved in hearing at 6 months F/U – At age two, eight of 33 infants were judged to be developing normally • A phase III placebo-controlled trial of ganciclovir is currently underway by the National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group (Kimberlin 2000) – Preliminary results suggest improvement/prevention of hearing loss during first 6-12 months of life and premature infants benefit most Treatment • Ganciclovir – Targets UL54 protein – Mutations in the UL97-encoded CMV phosphotransferase and alterations in viral DNA polymerase have been associated with resistance – DNA polymerase alteration also a/w cross-resistance to the nucleotide analog cidofovir • Valganciclovir • Foscarnet • Cidofovir – Nucleoside analogue • Treatment not usually indicated in immunocompetent patients • Indications – Treatment of disease in immunocompromised: retinitis, GI disease, pneumonitis, neurologic disease, viremia Treatment: CMV Retinitis Prevention • Behavioral Modification – Avoidance infectious saliva, urine, bodily fluids – Careful hygiene practices – Condom use www.med.nagoya-cu.ac Prophylaxis • Ganciclovir used for CMV prophylaxis in solid organ and allogenic bone marrow transplant patients post surgery • AIDS CMV prophylaxis – CD4 <50 • Ganciclovir not recommended by US PHS due to cost, lack of survival advantage, neutropenia, high pill burden • Ophtho exams Q 1-3 months – Immune reconstitution in response to ARV’s • Prophylaxis with antivirals x 6 months with CD4 >100 Vaccines • Live attenuated vaccine developed (Plotkin 1991) – Largest trial: Towne 125 strain – 500 subjects – Partial efficacy – Economically beneficial • Concerns – Reactivation and infection of host – Viral shedding from cervix or breast milk – Possible oncogenic potential of vaccine virus • Glycoprotein vaccine in guinea pig model (Bourne 2001) – Reduced in-utero CMV transmission – Improved pregnancy outcome