* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Natural History of Untreated HIV
Sarcocystis wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Herpes simplex wikipedia , lookup
Microbicides for sexually transmitted diseases wikipedia , lookup
Hepatitis C wikipedia , lookup
Marburg virus disease wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Epidemiology of HIV/AIDS wikipedia , lookup
Schistosomiasis wikipedia , lookup
Oesophagostomum wikipedia , lookup
Diagnosis of HIV/AIDS wikipedia , lookup
Neonatal infection wikipedia , lookup
Cryptosporidiosis wikipedia , lookup
Sexually transmitted infection wikipedia , lookup
Hepatitis B wikipedia , lookup
Coccidioidomycosis wikipedia , lookup
Herpes simplex virus wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
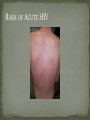
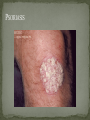
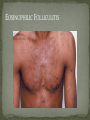
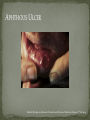
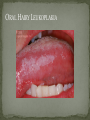
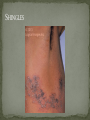
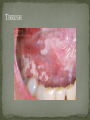
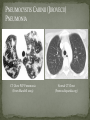
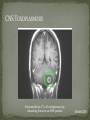
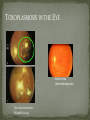
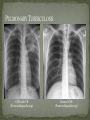
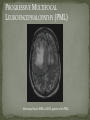
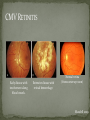
THE NATURAL HISTORY OF UNTREATED HIV-1 INFECTION PAUL ALLYN, MD AFRICAN AMERICAN HIV UNIVERSITY UNIVERSITY OF CALIFORNIA LOS ANGELES AUGUST 28, 2014 OBJECTIVES To illustrate the natural progression of untreated HIV-1 To highlight common clinical manifestations of HIV during this progression To discuss exceptions to this overall trend OVERVIEW Acute HIV-1 Infection Primary Infection Acute Retroviral Syndrome Clinical Latency Asymptomatic Disease Early Symptomatic Disease AIDS Clinical AIDS Advanced AIDS and Death CDC STAGING SYSTEM Stages based on CD4 cell count and symptoms. WHO CLINICAL STAGING SYSTEM Stage Description Stage 1 Asymptomatic or with persistent generalized lymphadenopathy, not AIDS. Stage 2 Minor mucocutaneous manifestations and recurrent upper respiratory tract infections, herpes zoster, mild weight loss (<10% of body weight). Stage 3 Unexplained chronic diarrhea, prolonged fever, severe bacterial infections, pulmonary tuberculosis, weight loss (>10% of body weight). Stage 4 PCP pneumonia, toxoplasmosis of the brain, esophageal candidiasis, Kaposi’s sarcoma, CMV, extrapulmonary TB, lymphoma, disseminated MAC, wasting syndrome, encephalopathy. Stages defined clinically, designed for resource-poor areas. TYPICAL COURSE OF HIV-1 INFECTION Adapted from Pantaleo et al. NEJM 1993 THE VIRUS Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases, 7th Ed. 2009. TRANSMISSION PRIMARY HIV INFECTION Timeframe: 0 weeks (immediately after transmission) Characterized by: High viral load (high concentration of HIV RNA in the blood) Declining CD4+ lymphocyte count (average about 1000 cells/mm3 prior to infection) Initially asymptomatic TYPICAL COURSE OF HIV-1 INFECTION Adapted from Pantaleo et al. NEJM 1993. ACUTE RETROVIRAL SYNDROME Timeframe: 1-6 weeks after exposure (peaks at 3 weeks) High viral load, low CD4 count Mononucleosis-like illness in 1/2 -2/3 of patients Symptoms typically resolve within 10-15 days Up to 50% patients asymptomatic ACUTE RETROVIRAL SYNDROME Symptoms variable in those who have them: Fever (96%) Enlarged lymph nodes (74%) Sore throat/Pharyngitis (70%) Rash (70%) Muscle or joint aches (54%) Low blood counts, platelets, and white cells (45%, 38%) Diarrhea (32%) Headache (32%) Nausea/Vomiting (27%) Hair loss (alopecia) Mood changes (depression, irritability) Data from Niu MT et al. JID 1993. RASH OF ACUTE HIV TYPICAL COURSE OF HIV-1 INFECTION Adapted from Pantaleo et al. NEJM 1993. CLINICAL LATENCY (ASYMPTOMATIC INFECTION) After acute infection, most patients remain asymptomatic for years Immune system develops antibodies to suppress the virus and the viral load stabilizes (viral set point) Over time, there is typically a gradual decline in CD4+ lymphocytes (on average 50-75 cells per year) Median time from infection to development of AIDS is approximately 8-10 years Some may develop AIDS in <5 years (approximately 20%) Few will remain asymptomatic without evidence of immunosuppression for more than 10 years (<5%) Many factors impact prognosis, but HIV-1 RNA levels (viral load) combined with CD4+ cell counts are the best predictor of disease progression to AIDS and death from AIDS PROBABILITY OF AIDS AT 3 YEARS ACCORDING TO CD4 CELL COUNT AND VIRAL LOAD Egger et al. Lancet 2002. PROBABILITY OF DEVELOPING AIDS BASED ON CD4+ LYMPHOCYTE COUNT AND VIRAL LOAD Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases, 7th Ed. 2009. WOMEN AND MEN: PROBABILITY OF SURVIVAL AT SAME CD4 COUNT >200 Female Male <=200 Female Male Chaisson RE et al. NEJM 1995. DOES EVERYONE DEVELOP AIDS IF LEFT UNTREATED? SPECIAL CIRCUMSTANCES Long-term nonprogressors: Remain asymptomatic without treatment or evidence of immunologic decline for many years 2 Groups: 1. Those with detectable viral load but adequate CD4+ cells to protect them from opportunistic disease (though these gradually decline over time) 2. Elite Controllers: Small group, have undetectable viral loads and maintain normal CD4+ lymphocyte counts Able to contain viral replication CLINICAL MANIFESTATIONS BY CD4 COUNT CD4+ COUNT >500 Patients with CD4+ counts > 500 generally asymptomatic May have mild or moderate lymphadenopathy (persistent generalized lymphadenopathy) Recurrent herpes infections may be present as well May have exacerbation of skin conditions: Psoriasis Eosinophilic folliculitis Aphthous ulcers Hairy Leukoplakia (benign white plaques on tongue) PSORIASIS EOSINOPHILIC FOLLICULITIS APHTHOUS ULCER Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases, 7th Ed. 2009. ORAL HAIRY LEUKOPLAKIA CD4+ COUNT 200-500 Most patients with CD4+ counts between 200 and 500 cells remain asymptomatic or have mild disease. May have: Worsening of chronic skin conditions Recurrent herpes simplex or varicella-zoster virus (shingles) Vaginal or oropharyngeal candidiasis (thrush) Recurrent diarrhea Intermittent fever Weight loss Muscle aches, joint aches, headache, and fatigue commonly reported Common to have bacterial sinusitis, bronchitis, pneumonia SHINGLES THRUSH TYPICAL COURSE OF HIV-1 INFECTION AIDS Adapted from Pantaleo et al. NEJM 1993. AIDS Patients with CD4+ Cells <200 are classified as having AIDS by 1993 CDC definition Certain opportunistic infections seen at this stage are indicative of AIDS, including: Pneumocystis carinii (jirovecii) pneumonia (PCP) Toxoplasmosis Cryptosporidiosis Esophageal candidiasis Tuberculosis Increased risk of certain cancers: Invasive cervical cancer in women Rectal or anal carcinoma in men Hematologic abnormalities (ITP, anemia, neutropenia) HIV-associated nephropathy (kidney disease) AIDS-DEFINING CONDITIONS Multiple or recurrent bacterial infections Candidiasis Invasive Cervical Cancer Coccidiomycosis, disseminated or extrapulmonary Cryptococcosis, extrapulmonary Cryptosporidiosis Cytomegalovirus disease Cytomegalovirus retinitis HIV-related encephalopathy Herpes simplex, chronic ulcers, bronchitis, pneumonitis, esophagitis Histoplasmosis, disseminated or extrapulmonary Isosporiasis, chronic intestinal Kaposi’s sarcoma Lymphoid interstitial pneumonia Burkitt’s lymphoma Immunoblastic lymphoma Primary CNS lymphoma Mycobacterium aviumintracellulare complex or M. kansasii, disseminated or extrapulmonary Mycobacterium tuberculosis, any site Pneumocystis carinii (jirovecii) pneumonia Recurrent pneumonia Progressive multifocal leukoencephalopathy Salmonella septicemia, recurrent Wasting syndrome of HIV infection CNS toxoplasmosis PNEUMOCYSTIS CARINII (JIROVECII) PNEUMONIA CT Chest PCP Pneumonia (From Mandell 2009) Normal CT Chest (From radiopaedia.org) CNS TOXOPLASMOSIS Abnormal brain CT with toxoplasma ringenhancing lesion in an AIDS patient. Mandell 2009 TOXOPLASMOSIS IN THE EYE Normal retina (from somerseye.com) Toxo chorioretinitis (Mandell 2009) PULMONARY TUBERCULOSIS CXR with TB (From radiopaedia.org) Normal CXR (From radiopaedia.org) END-STAGE AIDS Patients with CD4+ cells < 50 have end-stage immunodeficiency At risk for additional opportunistic illnesses: Disseminated Mycobacterium avium complex (MAC) Progressive multifocal leukoencephalopathy (PML) Cryptococcal meningitis Other disseminated fungal infections (coccidiomycosis, histoplasmosis, aspergillosis, Penicillium marneffei) Primary CNS lymphoma CMV Retinitis Wasting syndrome DISSEMINATED MAC Enlarged painless lymph node. Mandell 2009 PROGRESSIVE MULTIFOCAL LEUKOENCEPHALOPATHY (PML) Abnormal brain MRI in AIDS patient with PML. CMV RETINITIS Early disease with involvement along blood vessels. Extensive disease with retinal hemorrhage. Normal retina (from somerseye.com) Mandell 2009 CD4+ LYMPHOCYTE COUNT AT TIME OF DEVELOPMENT OF OPPORTUNISTIC ILLNESS Herpes simplex Herpes zoster (shingles) HIV Dementia Candida CMV esophagitis PCP Pneumonia Toxoplasmosis Disseminated MAC Moore RD and Chaisson RE. Ann Intern Med 1996. TYPICAL COURSE OF HIV-1 INFECTION AIDS Adapted from Pantaleo et al. NEJM 1993. AIDS DEATH Mean survival after reaching a CD4+ count of 200 is 38-40 months without treatment Mean survival after the development of clinicallydefined AIDS is 12-18 months (9 months in initial San Francisco cohort) Opportunistic infections independently increase risk of death OVERALL TRENDS CDC CAUSE OF DEATH IN THE PRE-HAART ERA: MACS 1984-1995 (2119 HIV+ PATIENTS) Unknown , 1.90% Non-AIDS Related, 5.70% AIDS-Related, 92.40% Overall Death Rate 9513 per 100,000 person years (General population 267) (Percentages are approximate to show general trend) Adapted from Wada N et al. Am J Epidemiol 2013. CAUSE OF DEATH IN THE HAART ERA: MACS 1996-2008 Unknown , 21.35% Non-AIDS Related, 21.35% Overall Death Rate 2842 per 100,000 person years (General population 463) AIDS-Related, 57.30% (Percentages are approximate to show general trend) Adapted from Wada N et al. Am J Epidemiol 2013. SUMMARY 1-6 weeks (average 3 weeks) after primary infection 1/2 to 2/3 of patients develop an acute mononucleosis-like illness called the acute retroviral syndrome that lasts 10-15 days. Following the acute infection, patients enter a period of clinical latency where they may remain mostly asymptomatic for up to 8-10 years on average, though this duration varies considerably. Disease progression can be predicted by baseline viral load and CD4+ cell count. Over time most patients (except for nonprogressors) will have declining CD4+ cells with increasing risk of developing symptoms. When CD4+ cells fall below 200 or with specific opportunistic infections, patients are defined as having AIDS. Risk of death increases dramatically when patients develop clinical symptoms of AIDS. HAART dramatically reduces this risk. KEY RESOURCES Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 7th Edition. Churchill Livingstone. 2009. Vergis EN and Mellors JW. Natural History of HIV-1 Infection. Infectious Disease Clinics of North America 2000. CDC: www.cdc.gov/hiv WHO: http://www.who.int/hiv/en/ QUESTIONS?