* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Electrons #1
Survey
Document related concepts
Quantum electrodynamics wikipedia , lookup
Particle in a box wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Spin (physics) wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Matter wave wikipedia , lookup
Wave–particle duality wikipedia , lookup
Tight binding wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
Ferromagnetism wikipedia , lookup
Molecular orbital wikipedia , lookup
Chemical bond wikipedia , lookup
Electron scattering wikipedia , lookup
Atomic theory wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Hydrogen atom wikipedia , lookup
Transcript
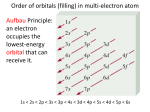
10/17/2012 Bellwork 1. Identify five things you can determine from this cell from the periodic table: 2. How many valence electrons does this element have? Grades Are you happy with your grade in Chemistry? Do you believe that there is anything that you can do to earn a better grade by the end of the semester? What can you do to improve your experience? Changes this Quarter Below a 70% on Tests & Quizzes Test & Quiz retakes End of semester projects ASP Availability Seating Charts Quote of the day... Its not about perfect. Its about effort. And when you bring that effort every single day, thats where transformation happens. That's how change occurs. Electron Configuration Review Neils Bohr - Electrons (e-) are in specific orbitals Protons = Electrons 2 electrons in orbit 1, 8 e- in orbit 2, 18 e- in orbit 3 Electron Configuration Electrons have little mass They exhibit particle & wave properties We can determine the position of an e- from the nucleus through 4 Quantum Numbers: 1. Principle Number (Energy of e-) 2. Angular Momentum Number (Shape of Orbital) 3. Magnetic Number (Orientation/Position of Orbital) 4. Spin Number (Spin on e- ; +/-) Principle Quantum Number (n) Distance from Nucleus Electrons far away have higher values for n Electrons that are closer are more attracted to the nucleus and are more stable As n increases, so does energy & the likelihood that the electron will be lost Angular Momentum (l) Angular Momentum Describes the shape of the orbital Can be shown by letters & numbers s, p, d, f and up the alphabet Angular Momentum (l) s orbital spherical in shape p orbital is dumbell in shape Angular Momentum (l) Another view Max of 2e- in s subshell Max of 6e- in p subshell Max of 10e- in d subshell Max of 14e- in f subshell Magnetic Number Shows the orientation in space s orbital p orbital Notation Physicist & Chemists use a standard notation to show the electron configurations of atoms: Hydrogen 1s1 (1 e- in s orbital of the 1st shell) Li 1s2 2s1 (2 e- in 1s subshell & 1 e- in the 2s subshell) P 1s2 2s2 2p6 3s2 3p3 Can you find the # of e- in Phosphorus? Notation In larger atoms, Noble Gasses are used to help abbreviate the notation Ex: We can change P from 1s2 2s2 2p6 3s2 3p3 to [Ne] 3s2 3p3 We can do this because Neon is 1s2 2s2 2p6 Find the closest Noble Gas & build from there Aufbau Principle The order for filling atomic orbitals Aufbau (German: to build up or construct) Note the pattern for s, p, d, & f s, p, d, f & the Periodic table The format of the periodic table relates to electron configuration Any Questions?