* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lacture №1. Chemical thermodynamics. The first law of
Survey
Document related concepts
Calorimetry wikipedia , lookup
Heat transfer wikipedia , lookup
Non-equilibrium thermodynamics wikipedia , lookup
Thermal conduction wikipedia , lookup
Heat transfer physics wikipedia , lookup
Conservation of energy wikipedia , lookup
First law of thermodynamics wikipedia , lookup
Internal energy wikipedia , lookup
Second law of thermodynamics wikipedia , lookup
Gibbs free energy wikipedia , lookup
Adiabatic process wikipedia , lookup
History of thermodynamics wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Transcript
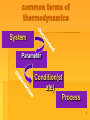
Chemical thermodynamics. The first law of thermodynamics. Plan 1 The basic concepts of thermodynamics 2. First law of thermodynamics. Heat (Q) and Work ( W) 3. Heat capacity Assistant Kozachok S.S. prepared 1 The branch of science which deals with energy changes in physical and chemical processes is called thermodynamics Some common terms which are frequently used in the discussion of thermodynamics are: 2 common terms of thermodynamics System Parameter Condition(st ate) Process 3 Classification of the thermodynamics systems according to a structure homogeneous KNO3 heterogeneous KNO3 PbI2↓ 4 System is a specified part of the universe which is under observation The remaining portion of the universe which is not a part of the system is called the surroundings The system is separated by real or imaginary boundaries. 5 Types of Systems ISOLATED Open Close (a system which can not exchange mass and energy with the surroundings ) CLOSE A system which can exchange energy but no mass with its surroundings 6 Parameters Extensive Intensive (m, V, U, H, G, S, c) The properties of the system whose value depends upon the amount of substance present in the system (p, T, C, viscosity, surface tension, vapour pressure) The properties of the system whose value does not depend upon the amount of substance present in 7 the system Process is the change of all or individual parameters of the system during the length of time (the period of time) Classification of a process according to the constant parameter of a system are: Isothermic process – temperature is constant, T=const Isochoric process – volume is constant V = const. Isobaric process – pressure of the system is constant, p = const Adiabatic process – the system is completely isolated from the surroundings. For an adiabatic (Q=0) system of constant mass, ▲U=W 8 Classification of a process according to the releasing energy Exothermic process is a process that releases energy as heat into its surroundings. We say that in an exothermic process energy is transferred ‘as heat’ to the surroundings. For example: a reaction of neutralization (acid + basic). Endothermic process is a process in which energy is acquired from its surroundings as heat. Energy is transferred ‘as heat’ from the surroundings into the system. For example: the vaporization of water 9 Classification of a process according to the direction of reaction Reversible process is a process in which the direction may be reversed at any stage by merely a small change in a variable like temperature, pressure, etc. Irreversible process is a process which is not reversible. All natural process are irreversible 10 State of a system means the condition of the system , which is described in terms of certain observable (measurable) properties such as temperature (T), pressure (p), volume (V) State function (thermodynamic function) Internal energy U [J/mol] Enthalpy H [kJ/mol] or [kJ] Entropy S [J/mol K] or [J/K] Gibbs energy G [J/mol] or [J] ΔU = Uproducts - Ureactants 11 State function depends only upon the initial and final state of the system and not on the path by which the change from initial to final state is brought about. 12 Internal energy U It is the sum of different types of energies associated with atoms and molecules such as electronic energy, nuclear energy, chemical bond energy and all type of the internal energy except potential and kinetic energies. 13 Heat (Q) is a form of energy which the system can exchange with the surroundings. If they are at different temperatures, the heat flows from higher temperature to lower temperature. Heat is expressed as Q. 14 Work (W) is said to be performed if the point of application of force is displaced in the direction of the force. It is equal to the distance through which the force acts. There are two main types of work electrical and mechanical. Electrical work is important in systems where reaction takes place between ions. Mechanical work is important specially in systems that contains gases. This is also known as pressure-volume work. 15 The work of the expanded ideal gass W Fdh F ( force) p( pressure ) S (area ) WORK = Force and Distance F s h A pSdh pdV 2 A pdV 1 16 If the volume of the system changes by a finite quantity from volume V1 to V2, then total work done can be obtained by integrating: If the gas expands, V2 › V1 and work is done by the system and W is negative (we will use sign positive) V2‹ V1 and the work is done on the system and W is positive Note. It may be noted that many books use the opposite sign convention for work!!! (according to the IUPAC recommendation) 17 Enthalpy H Chemical reactions are generally carried out at constant pressure. ΔU gives the change in internal energy at constant volume. To express the energy changes at constant pressure, a new term called enthalpy was used. Enthalpy cannot be directly measured, but changes in it can be. 18 Enthalpy H A thermodynamic function of a system, equivalent to the sum of the internal energy of the system plus the product of its volume multiplied by the pressure exerted on it by its surroundings. ▲H = ▲U + p▲V 19 The meaning of ▲H and ▲U 1) ▲H = ▲U in solid or liquid systems 2) ▲H >>▲U in gas system 20 Heat absorbed by the system = H positive (Q negative Heat evolved by the system = H negative (Q positive) The signs of W or Q are related to the internal energy change. The meaning of the state functions in the thermodynamic processes Exothermic process Qv > 0, ▲U < 0 Qp > 0, ▲H < 0 2) Endothermic process Qv < 0, ▲U > 0 Qp < 0, ▲H > 0 21 The first law of thermodynamics Energy can neither be created nor destroyed although it may be converted from one form to another. The given heat for the system spents on the change of the internal energy and producing the work: Q = ▲U + W The internal energy of the system can be changed In two ways: a) By allowing heat to flow into the system or out of the system b) By work done on the system or work done by the system. 22 Determination according to the process: 1) Isochoric process, V-constant W= p▲V (▲V = V2- V1) = 0 Qv = ▲U; 2) Isobaric process, p-constant Qp = ▲U + W , Qp = ▲U + p▲V , W=nRT (according Mendeleyev Klapeyron equation V=nRT/P) Qp = ▲ H 3) Isothermic process, T – constant ▲U = n Cv ▲T = 0 QT = W =nRT lnV2/V1 = nRT lnP1/P2 23 24