* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download IB Physics
Survey
Document related concepts
Thermal conduction wikipedia , lookup
Calorimetry wikipedia , lookup
Conservation of energy wikipedia , lookup
Equation of state wikipedia , lookup
Heat transfer physics wikipedia , lookup
First law of thermodynamics wikipedia , lookup
Thermal expansion wikipedia , lookup
Internal energy wikipedia , lookup
Second law of thermodynamics wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Gibbs free energy wikipedia , lookup
Thermodynamic system wikipedia , lookup
History of thermodynamics wikipedia , lookup
Transcript
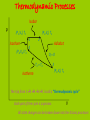
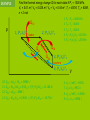
IB Physics Topic 10 – Thermodynamic Processes Mr. Jean The plan: • Video clip of the day – https://www.youtube.com/watch?v=nBVL3lp9f Go – https://www.youtube.com/watch?v=s3tjOEtoAr g • Returning Exams • Thermodynamic processes Returning Exams: • Review your exams • If you have any questions we can go over some of the solutions briefly. Gravitational Waves: • https://www.youtube.com/watch?v=gwi_VKd6Wo Combining the gas laws gives PV = (a constant)×T Therefore, for n moles of an ideal gas; P = Pressure PV = nRT V = Volume n = number of moles R = Universal gas constant T = Temperature (KELVIN) Deduce an expression for the work involved in a volume change of a gas at constant pressure. The work done by this force is w = Fs = PAs, since F=PA but As is the change in the volume occupied by the gas, ΔV. therefore; W = PDV State the first law of thermodynamics. We can add energy to a gas by heating Q (temperature gradient) Or by working (mechanical energy) = W Q = ΔU + W Q = Heat energy added to the ΔU = Internal energy increase of the gas W = Work done by the gas. Students should be familiar with the terms system and surroundings. They should also gas appreciate that if a system and its surroundings are at different temperatures and the system undergoes a process, the energy transferred by nonmechanical means to or from the system is referred to as thermal energy (heat). 1. Change of p (and T) at constant volume; an isovolumetric change. 2. Change of V (and T) at constant pressure; an isobaric change. 3. Change in p and V at constant temperature; an isothermal change. 4. Change in p and V in an insulated container (no heating of the gas); an adiabatic change. The product of pressure and volume represents a quantity of work. This is represented by the area below a p-V curve. Therefore, the area enclosed by the four curves represents the net work done by the engine during one cycle. Second Law of Thermodynamics: It is impossible to extract an amount of heat QH from a hot reservoir and use it all to do work W . Some amount of heat QC must be exhausted to a cold reservoir. Entropy is a measure of the disorder (of the energy) of a system Every time we change energy from one form to another, we increase the entropy of the Universe even though local entropy may decrease. Thermodynamic Processes A system can change its state A state is a unique set of values for P, V, n, & T (so PV = nRT is also called a “State Equation”) When you know the state of a system you know U since U = NkT = nRT = PV, for a monatomic gas A “process” is a means of going from 1 state to another There are 4 basic processes with n constant “iso” means “same” Isobaric, a change at constant pressure Isochoric or isovolumetric, a change at constant volume, W = 0 Isothermal, a change at constant temperature (DU = 0, Q = W) Adiabatic, a process is one in which no heat is gained or lost by the system. Thermodynamic Processes Isobar P (P1,V1) T1 (P2,V2) T2 1 Isochore (P4,V4) T4 2 Adiabat 4 Q=0 T3 = T4 Isotherm 3 (P3,V3) T3 The trip from 12341 is call a “thermodynamic cycle” Each part of the cycle is a process V All state changes can be broken down into the 4 basic processes Thermodynamic Processes Isobar, expansion at constant pressure, work is done P 1 Isochoric 4 pressure change, W = 0 2 Adiabatic expansion; no heat, Q = 0 3 Isothermal compression W = Q, U is constant The area enclosed by the cycle is the total work done, W The work done, W, in a cycle is + if you travel clockwise V Heat Engines and Refrigerators Engines use a working fluid, often a gas, to create motion and drive equipment; the gas moves from 1 state (P, V, n, & T define a state) to another in a cycle The Stirling Cycle: 2 isotherms 2 isochores Stirling designed this engine in the early 18th century – simple and effective The Stirling Engine Isobaric expansion of a piston in a cylinder The work done W = Fd = PAd = PDV The work done is the area under the process W = PDV 4 stroke engine Isochoric expansion of a piston in a cylinder The work done W = 0 since there is no change in volume Thus DU = Q – W = Q Adiabatic expansion of an ideal gas The work done W = 0 here because chamber B is empty and P = 0 Thus DU = Q – W = 0, that is adiabatic expansion against no resistance does not change the internal energy of a system EXAMPLE How much work is done by the system when the system is taken from: (a) A to B (900 J) (b) B to C (0 J) (c) C to A (-1500 J) Each rectangle on the graph represents 100 Pa-m³ = 100 J (a) From A B the area is 900 J, isobaric expansion (b) From B C, 0, isovolumetric change of pressure (c) From C A the area is -1500 J EXAMPLE 10 grams of steam at 100 C at constant pressure rises to 110 C: P = 4 x 105 Pa DT = 10 C DV = 30.0 x 10-6 m3 c = 2.01 J/g What is the change in internal energy? DU = Q – W DU = mcDT – PDV DU = 189 J So heating the steam produces a higher internal energy and expansion EXAMPLE Aluminum cube of side L is heated in a chamber at atmospheric pressure. What is the change in the cube's internal energy if L = 10 cm and DT = 5 °C? DU = Q – W Q = mcDT m = V0 V0 = L3 W = PDV DV = V0DT DU = mcDT – PDV cAl = 0.90 J/g°C DU = V0cDT – PV0DT Al = 72(10-6) °C-1 DU = V0DT (c – P) Patm = 101.5 kPa Al = 2.7 g/cm³ DU = L³DT (c – P) DU = 0.10³(5)((2700)(900) – 101.5(10³)(72(10-6)) DU = 12,150 J NB: P is neglible EXAMPLE Find the work done for a cycle if P1 = 1000 kPa, V1 = 0.01 m³, V2 = 0.025 m³, V3 = V4 = 0.04 m³, T1 = 400 K, T2 = 600K, n = 2 mol W = Area enclosed = P1DV12 + (P2+P3)DV23 – (P1+P4)DV41 P 1, (P1,V1) T1 Isobar 2, (P2,V2) T2 3, (P3,V3) T3 Isochore 4, (P4,V4) T4 1. P2 = P1 = 1000 kPa 2. T4 = T1 = 400 K 3. T3 = T2 = 600 K 4. P3 = P2V2/V3 = 625 kPa 5. P4 = P1V1/V4 = 250 kPa V W = Area enclosed = P1DV12 + (P2+P3)DV23 + (P1+P4)DV41 = (15 + 12.188 – 18.75)(10³) = 8.44 kJ EXAMPLE P Find the internal energy for each state if P1 = 1000 kPa, V1 = 0.01 m³, V2 = 0.025 m³, V3 = V4 = 0.04 m³, T1 = 400 K, T2 = 600K, n = 2 mol 1, (P1,V1) T1 Isobar 2, (P2,V2) T2 1. P2 = P1 = 1000 kPa 2. T4 = T1 = 400 K 3. T3 = T2 = 600 K 4. P3 = P2V2/V3 = 625 kPa 5. P4 = P1V1/V4 = 250 kPa 3, (P3,V3) T3 Isochore 4, (P4,V4) T4 6. U1 = nRT1 = 9972 J 7. U4 = U1 = 9972 J 8. U2 = nRT2 = 14958 J 9. U3 = U2 = 14958 J V EXAMPLE Find the thermal energy change Q for each state if P1 = 1000 kPa, V1 = 0.01 m³, V2 = 0.025 m³, V3 = V4 = 0.04 m³, T1 = 400 K, T2 = 600K, n = 2 mol Q12 P 1, (P1,V1) T1 Isobar 2, (P2,V2) T2 1. P2 = P1 = 1000 kPa 2. T4 = T1 = 400 K 3. T3 = T2 = 600 K 4. P3 = P2V2/V3 = 625 kPa 5. P4 = P1V1/V4 = 250 kPa Q34 Q41 Q34 3, (P3,V3) T3 Isochore 4, (P4,V4) T4 10. Q12 = DU12 + W12 = 34986 J 11. Q23 = W23 (DU23 = 0) W23 = (P2+P3)DV23 = 12.188 kJ 12. Q34 = DU34 = -4986 J 13. Q41 = W41 (U41 = 0) W41 = (P4+P1)DV41 = - 18.75 kJ V 6. U1 = nRT1 = 9972 J 7. U4 = U1 = 9972 J 8. U2 = nRT2 = 14958 J 9. U3 = U2 = 14958 J Heat Engines and Refrigerators The Wankel Rotary engine is a powerful and simple alternative to the piston engine used by Nissan and invented by the German, Wankel in the 1920s The Wankel Cycle: 2 adiabats 2 isochores The Wankel Engine