* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Medical Genetics 1
Gene desert wikipedia , lookup
Genetic engineering wikipedia , lookup
Saethre–Chotzen syndrome wikipedia , lookup
Gene nomenclature wikipedia , lookup
Point mutation wikipedia , lookup
Hardy–Weinberg principle wikipedia , lookup
Frameshift mutation wikipedia , lookup
Gene expression programming wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Medical genetics wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Gene therapy wikipedia , lookup
Quantitative trait locus wikipedia , lookup
Tay–Sachs disease wikipedia , lookup
Genome-wide association study wikipedia , lookup
Fetal origins hypothesis wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Population genetics wikipedia , lookup
Genetic drift wikipedia , lookup
Designer baby wikipedia , lookup
Genome (book) wikipedia , lookup
Dominance (genetics) wikipedia , lookup
Public health genomics wikipedia , lookup
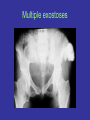
Medical Genetics 1 Prof Duncan Shaw http://www.abdn.ac.uk/~gen155/djshome.html Major Groups of Clinical Disorders with a Genetic Contribution • • • • Single gene defects Chromosomal abnormalities Congenital malformations Multifactorial diseases - most common causes of illness Autosomal recessive inheritance • Cystic fibrosis (1/2000) • Recessive mental retardation (1/2000) • Congenital deafness (1/5000) Increased risk in autosomal recessive disease • Consanguinity: if parents are related (consanguinity) there is an increased risk that both parents carry the same recessive allele Degree Example % alleles shared 1st Siblings, parent/child 50 2nd Uncle/niece, grandparent/child 1st cousins 25 3rd 12.5 Ethnic associations with AR disease • In particular populations, recessive allele frequency may have increased by selection in heterozygotes, or by genetic drift • -Thalassaemia: Cypriots, Greeks, Italians, Chinese, African-Americans • Sickle Cell Disease: Arabs, West Indians • Tay-Sachs Disease: Ashkenazi Jews (4% carriers) • Severe Combined Immunodeficiency Syndrome: Apache Native Americans • Cystic Fibrosis: Caucasians Finding the cystic fibrosis gene • CF gene was found using positional cloning • Linkage to markers on chromosome 7 • But that didn’t get closer than several Mb – still lots of genes • To narrow the candidate region further, used linkage disequilibrium….. Linkage and linkage disequilibrium • Linkage is tested within families, LD by population study • This marker is linked to the disease, but to different alleles (of the same marker gene) in each family 1,2 1,1 1,2 1,1 2,2 1,1 1,2 2,2 1,2 2,2 1,1 2,2 How LD arises LD and haplotypes • Haplotype – the set of alleles carried by an individual chromosome • With N bi-allelic markers, expect 2N possible haplotypes in population, because recombination creates all possible combinations of alleles • If fewer than 2N haplotypes are observed, this is evidence for LD • Previous example: A1/A2 and CF/N gives 4 haplotypes with recombination, or 3 with LD Testing for LD Patients Marker A (shows LD with disease) Marker B (no LD, but could be linked) Allele 1 Allele 2 Allele 1 Allele 2 150 50 120 80 100 120 80 Controls 100 c2 test for significance LD operates over short genetic distances 1 LD 0 -5000 -100 0 +100 Distance (kb) from disease gene +5000 Use of LD for gene mapping • A gene can be mapped by linkage in families to within a few cM ( = a few Mb in humans) • If all or most cases of the disease are descended from a unique mutation, LD will be observed with markers about 100kb or less from the gene – much closer than you can get using linkage alone • In CF, about 70% of mutations are the same (DF508) and these show LD with markers very close to the CF gene – this helped the gene to be identified Autosomal dominant inheritance • An affected person usually has one affected parent • Transmitted by either sex • Child of an affected parent is at 50% risk of also being affected Autosomal Dominant Diseases Disease: Frequency/1000 births: Otosclerosis Familial hypercholesterolaemia Adult polycystic kidney disease Multiple exostoses Huntington’s disease 3 2 1 0.5 0.4 Multiple exostoses The ear Comparisons between AD and AR Dominant Recessive • Expressed in heterozygote • Approx. 1/2 offspring affected • Equal frequency and severity in each sex • Paternal age effect on rate of new mutation • Variable expressivity • Expressed in homozygote • Low risk to offspring • Equal frequency and severity in each sex • New mutations rare • Constant expressivity in each family • Importance of consanguinity Revision of linkage and Lod scores • Affecteds have A marker allele from Dad, unaffecteds have B • If random, would expect 50:50 distribution • Evidence for linkage? Revision of linkage and Lod scores (2) • If marker and disease were unlinked, probability of this pedigree: (1/2)4 = 1/16 = 0.0625 • If they are linked with RF = 0.1 (10% recombination), probability of pedigree: (0.9)4 = 0.66 and odds ratio (relative to no linkage) = 0.66/0.0625 = 10.56 • If they are linked with RF = 0.0, probability of pedigree: (1)4 = 1 and odds ratio (relative to no linkage) = 1/0.0625 = 16 • To combine information from several families, take log10 of odds ( = LOD score) and add them up • LOD > 3 good evidence for linkage; LOD < -2 evidence against linkage; -2 < LOD < 3 is inconclusive