* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Protein Calorie Malnutrition
Survey
Document related concepts
Protein–protein interaction wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Citric acid cycle wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Proteolysis wikipedia , lookup
Phosphorylation wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Blood sugar level wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Transcript
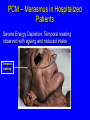
Protein Calorie Malnutrition Protein-Calorie Malnutrition • PCM affects ~ 1 billion individuals world-wide • In US, 30-50% of patients will be malnourished at admission to hospital • 69% will have a decline in nutrition status during hospitalization • 25-30% will become malnourished during hospitalization Malnutrition in Hospitalized Pts • Consequences for hospitalized pts: – – – – – poor wound healing higher rate of infections greater length of stay greater costs Increased morbidity and mortality Definitions • Fast: exclusion of all food energy • Starvation: prolonged inadequate intake of protein and/or energy • Cachexia: wasting induced by metabolic stress Brief Review of Fed State • Exogenous fuel utilization • Absorption of glucose and amino acids stimulates insulin secretion • Deposition of nutrients in tissue – Glucose: glycogen, triglyceride synthesis – Amino Acids: protein synthesis, mainly in muscle Fuels in Fed State • Glucose-dependent: brain, blood cells and renal medulla – Brain uses 50% of available glucose • Preferential users of glucose: heart, renal cortex and skeletal muscle • Fatty acids: liver • Protein/AA: not used as fuels unless excessive intake Postabsorptive State • Fed state ends when last nutrient is absorbed, body switches to endogenous fuel utilization • Decrease level of insulin, increase in glucagon – Release, transfer and oxidation of fatty acids – Release of glucose from liver glycogen – Release of free amino acids from muscle as a source of fuel Progression of Fasting • Normal post-absorptive state: 12 hours – Draw on short term reserves to maintain blood glucose levels for glucose-dependent tissues (brain, blood cells, and renal medulla) • release and oxidation of fatty acids • release of glucose from liver glycogen – Liver glycogen capacity: approximately 1000 kcal – Equivalent to 250g carbohydrate/glucose Fast Longer than 24 hours • Further decrease in insulin, increase in glucagon • Proteolysis and release of amino acids from muscle as a source of fuel • Activation of hormone sensitive lipase – increase in lipolysis – increase in circulating FFA and TG • Gluconeogenesis increases Gluconeogenesis • Cori cycle in Liver – glucose --> converted to lactate/pyruvate in skeletal muscle (anaerobic)-->travels back to liver for conversion to glucose Gluconeogenesis • Glucose-Alanine Cycle: Liver – AA deaminated in muscle – C-skeleton used for energy -->pyruvate and NH2 --> alanine – alanine returns to liver for deamination – NH2 -->urea for excretion – pyruvate --> glucose via GNG Gluconeogenesis • Glutamine cycle in Kidney – Muscle glutamine --> kidney --> glutamate + NH3 -->a-ketoglutarate --> glucose • Kidney is initially a minor source, over time increases to supply up to 50% of glucose Fast longer than 2-3 days • GNG ongoing, sources of substrate: – endogenous glycerol – alanine and glutamine from muscle – lactate and pyruvate • Ketosis Fast longer than 2-3 days • Ketosis – characterized by presence of ketone bodies • acetoacetate, acetone, b-hydroxybutyrate – byproduct of fatty acid oxidation in liver – can be used by all tissues with mitochondria – utilized by brain, decreasing glucose consumption by 25% – Can be prevented by providing 150g glucose per day Fast longer than 2-3 days • Significant protein loss during first 7-10 days – Body protein losses: • • • • 10-12 g urinary N/day 360 g LBM per day initially 1-2 kg LBM over first 7 days Lethal depletion after 3 weeks if no adaptation occurs - by the end of 2-3 weeks, decrease muscle protein catabolism to <1/3 of initial (not yet understood) Long Term Starvation (>7-10d) • Decreased metabolic rate – decreased activity, body temperature • Conservation of protein – decrease in muscle pro breakdown from 75g to 20 g per day • Increased fatty acid oxidation – Liver, heart and muscle use ketone bodies Long Term Starvation (>7-10d) • Decreased glucose availability – Brain: – – – – – fed state: uses 75% (140g/day), completely oxidized >3 week of fast: replace 50% of glucose with ketones decreased complete oxidation, recycles via GNG Blood cells/Renal medulla anaerobic glycolysis to pyruvate and lactate Origin of blood glucose: (I) Exogenous; (II) Glycogen, Liver gluconeogenesis; (III) Liver gluconeogenesis, Glycogen; (IV & V)Liver and Kidney gluconeogenesis Major fuel of brain: (I) - (III) Glucose; (IV) Glucose, ketone bodies; (V) Ketone bodies, glucose Minnesota study (1944-1946) • 32 young, healthy “volunteers” consumed 2/3 of normal energy intake (1600 kcal) for 24 weeks • wt loss of 23% of body weight – loss of 70% of fat mass – loss of 24% of lean body mass • wt loss alone underestimated loss of body mass due to increase in edema Minnesota study (1944-1946) • Decrease in metabolic rate by 40% – – – – corresponds to decreased in food energy correlates to loss of lean body mass reduced per unit of remaining LBM lower thermal effect of food due to smaller meals – decrease in physical activity – achieve new “energy balance” Starvation • Functional alterations – hormonal changes • decreased thyroid fx --> decreased BMR • decreased gonadotropins • decreased somatomedins --> decreased muscle/cartilage synthesis, decreased growth – decreased metabolic rate and caloric need – decreased body temp – decreased activity, increased sleep Starvation • Changes in Organ Function – GI tract - loss of mass, decreased villi and crypts – decreased enzyme secretion – impaired motility – tendency for bacterial overgrowth – maldigestion and malabsorption Starvation • Changes in Organ Function – Liver: loss of mass • decreased protein synthesis • periportal fat accumulation (fatty liver) • hepatic insufficiency – Skeletal muscle • catabolized for GNG - decreased mass • utilization of ketones: slower contractions • diminished function: intercostal muscles - decreased respiratory function Starvation • Changes in Organ Function – Cardiovascular system • • • • decreased cardiac output bradycardia, hypotension dilatation, degeneration, fibrosis central circulation takes precedence, leads to postural hypotension – Respiratory system: • decreased cilia, reduced bacterial clearance • decreased deep breathing Starvation • Changes in Organ Function – Kidney • decreased perfusion, decreased GFR • increased GNG • increased NH4 excretion – Immune function • • • • decreased T-lymphocyte count decreased cytokine activity anergy increased infection rate (pneumonia) Starvation • Changes in Organ Function – Nervous system: • decrease in nerve myelination • decrease brain growth Successful Adaptation • Goals: 1. Maintain glucose homeostasis and conserve glucose pool. 2. Preserve structural and functional lipids and proteins 3. Preserve the organism Preferential visceral uptake of AA released by peripheral tissue Failed adaptation • Metabolic disease: hyperthyroidism/thyroid storm, insulinoma • Micronutrient deficiency - mineral deficiency interferes with protein sparing • Food restriction too severe • Metabolic stressors such as infection, surgery lead to “hypermetabolic state” Hypermetabolic State and Cachexia • Wounds, surgical stress, cancer, inflammatory conditions and infection Increased production of cortisol, interleukins, TNF hypercatabolic state with increased RMR = increased energy requirements Insulin resistance, hyperglycemia - no starvation adaptation, poor utilization of stubstrate • Protein breakdown continues unabated In some burn patients amount of protein catabolized can reach 200 g/d = ~0.5 lb/day lean body mass! Severe protein malnutrition results in as little as 1 week. Repletion of body stores is not achievable until metabolic stressor has been resolved PCM: Clues to Cause From Body Composition Analysis • Energy depletion (reduced fat stores) out of proportion to LBM loss: Starvation = Marasmus • Predominant protein depletion (reduced LBM): Cachexia = Kwashiorkor • Combined (Marasmic Kwashiorkor): Most common PCM seen in hospitalized patients PCM – Marasmus in Hospitalized Patients Severe Energy Depletion: Temporal wasting observed with ageing and reduced intake Temporal wasting PCM – Marasmus in Hospitalized Patients Severe Energy Depletion: Loss of Skinfold Thickness Nutrition Assessment Hospital or Clinic Screening • Identifying and treating malnutrition • Preventing Hospital-Acquired Malnutrition • Assessing nutrition risk on admission: JCAHOmandated database • more to come...