* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lecture_5a_ Catalysis . ppt - University of Massachusetts
Survey
Document related concepts
Basal metabolic rate wikipedia , lookup
Radical (chemistry) wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Proteolysis wikipedia , lookup
Glass transition wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Biochemistry wikipedia , lookup
Biosynthesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Transcript
Enzyme Catalysis
Bill Royer
Office: LRB 921
Phone: x6-6912
Enzymes have spectacular abilities to accelerate chemical reactions –
often by factors of 106-1014 over non-catalyzed reactions. In this
lecture, we will briefly discuss some of the strategies used by enzymes
to achieve such remarkable rate increases.
I. Transition state theory
II. Mechanisms of catalysis
Acid-base catalysis - Ribonuclease A
Metal ion catalysis - Hammerhead Catalytic RNA
Covalent catalysis - Chymotrypsin
I. Transition state theory
Consider the reaction A + B P + Q
A+B
K‡
‡
k'
P+Q
where A + B react through transition state, X‡, to form products P + Q. K‡ is the
equilibrium constant between A + B and X‡ and k' is the rate constant for
conversion of X‡ to P + Q.
‡
G ‡
G
A+B
G reaction
P+Q
Reaction coordinate
The minimum energy pathway of the reaction
is shown in the reaction coordinate, or
transition state diagram, at left. Chemical
conversion of A + B to P + Q proceeds
through a transition state ‡ which is the
least stable (least probable, highest free
energy) species along the pathway.
Molecules that achieve the activation energy,
G‡ , can go on to react while molecules that
fail to achieve the transition state fall back to
the ground state.
The transition state, X‡, is metastable. (Unlike a reaction intermediate, the transition
state has only a transient existence, like a pebble balanced on a pin. By definition, a
transition state cannot be isolated.) The transition state can be thought of as sharing
some features of the reactants and some features of the products. That is, some
bonds in the substrate are on their way to being broken and some bonds in the
product are partially formed.
The transition state, X‡, is in rapid equilibrium with reactants
with equilibrium constant K‡.
‡
K‡
[A] [ B]
G‡, the activation energy, is the difference in Gibbs free energy between the transition
state, X‡, and the reactants. Since K‡ is an equilibrium constant, the now familiar
equation applies:
‡
‡
-RT lnK
= G
where T is the absolute temperature in degrees Kelvin (°C + 273) and R is the gas constant
(1.98 cal / mol / degree). In other words, the frequency with which reactants achieve the
transition state is inversely proportional to the activation energy barrier between the two.
The observed rate of the reaction, kobs, will be a
function of the concentration of the reactants, the rate
of conversion of X‡ to P + Q, k', and will decrease
exponentially with an increase in G‡.
k obs = k' e
-G‡ / RT
[A][B]
Thus, the smaller the difference in free energy of the reactants and the transition state, the faster
the reaction proceeds. Enzymatic rate accelerations are achieved by lowering the activation
barrier between reactants and the transition state, thereby increasing the fraction of
reactants able to achieve the transition state. Enzymes reduce the activation barrier by
destabilizing the ground state of enzyme-bound substrates and products, by stabilizing the
transition state, and/or by introducing a new reaction pathway with a different transition state that
has a lower free energy.
‡
Uncatalyzed
Enzymes accelerate reactions by lowering the
energy barrier between reactants and products.
‡
Gcat
G‡ = G‡uncatalyzed - G‡catalyzed
G
A+B
Catalyzed
A+B
P+Q
P+Q
Reaction coordinate
Although less energy is required to form the
transition state in the catalyzed reaction, the
ground states of the free substrates and products
remain the same. The kinetic barrier is lowered
by the same extent for the forward and reverse
reactions. Consequently, a catalyst accelerates
the reaction without affecting its equilibrium .
If a catalyst lowers the activation barrier by G‡, the rate of the reaction is enhanced
by the factor e G‡/RT. Consequently, a ten-fold rate enhancement requires that G‡ =
1.36 kcal/mole, less than the energy of a single hydrogen bond.
(G‡ = RTln10 = 1.98 x 10-3 kcal/mol*K x 298K*ln(10) = 1.36 kcal/mol)
Imaginary enzyme ("stickase") designed to catalyze "cleavage" (breaking) of a metal stick
(Nelson & Cox, Lehninger Principles of
Biochemistry, 3rd ed., 2000)
For a reaction that involves several steps, each step will have a corresponding
transition state.
k1
AI
A‡
G
I‡
I
P
1
kk11<> kk22
I
k1
the formation of I, an intermediate, from A is
kk11 >< kk22 If
slower than the formation of P from I (k < k )
A
A
k2
k2
Reaction coordinate
P
P
2
the activation barrier for the first step must be
higher than the activation barrier for the second
step (thick line). If k 1is much slower than k , 2
conversion of A to I is the rate-determining step
for the reaction. That is, the overall reaction
proceeds at a rate that can be no faster than k . 1
Conversely, if formation of P from I is much
slower than formation of I from A (k <2k ),1the
activation barrier for the second step is higher
(thin line) and formation of P from I is
rate-determining.
II. Mechanisms of catalysis
A. Acid-base catalysis
Specific acid or base catalysis - Reaction rate is directly proportional to [H+] or [OH-].
Example: Alkaline hydrolysis of RNA
General acid or base catalysis - Reaction rate is proportional to [Bronsted acid] or
[Bronsted base]
Bronsted acid - species that can donate protons
Bronsted base - species that can combine with a proton
Specific Base Catalysis
General Base Catalysis
Rate
Rate
pH 7.3
pH 7.0
pH 7.3
pH 7.0
[Imidazole buffer]
[Imidazole buffer]
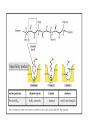
Amino acids side chains with pKa's in the neutral pH range can
function as Bronsted acids/bases
Amino Acid
Aspartic acid
pK a
3.90
-COOH
COO -
O
H C CH 2 C
ONH 3+
-COOH
COO Glutamic acid
4.07
O
H C CH 2
NH 3+
CH 2 C
O-
COO Histidine
6.04
H C CH
NH 3+
imidazole
N
2
N
COO Cysteine
8.33
H C CH
NH 3+
sulfhydryl
2
SH
phenol
COO Tyrosine
10.13
H C CH
NH 3+
OH
2
-amino
COO Lysine
10.79
H C CH 2
NH 3+
CH 2
CH2 NH +
3
Biologically important
nucleophilic groups:
Nucleophilic
form
Hydroxyl group
R-OH
R-O:
Sulfhydryl group
R-SH
R-S: -
+ H+
Amino group
R-NH3+
R-NH2
+ H+
R
Imidazole group
HN + NH
+ H+
R
HN
N:
+ H+
Biologically important
electrophiles:
H+
Protons
Mn+
Metal Ions
C=O
Carbonyl carbon
R-NH2
+ C=O
Adapted from
Voet & Voet,
Biochemistry
Ribonuclease A
An example of concerted acid-base catalysis - reaction subject to both
general acid and general base catalysis
5'... O
5'... O
O Pyrimi dine
5'... O
O
O Pyrimi dine
Pyrimi dine
H2 O
O OH
O P O
O
O
O O
P
O O-
2',3'-Cyclic phosphate
Bas e
H
O OH
O P O
O
3' phosphate
HO
O
Bas e
OHOH
3'...O OH
RNase A (124 residues, mw 13.7 kd) is a digestive enzyme secreted by the pancreas
that catalyzes hydrolysis of phosphodiester backbone of RNA. In first step of the
reaction, cleavage of the bond between phosphorous and the 5' oxygen generates one
2',3'-cyclic phosphate terminus and one 5'-OH. In the second step, water reacts with
the cyclic phosphate to yield a 3' phosphate. The 2',3' cyclic phosphate can be
isolated because it forms more rapidly than it hydrolyzes.
First Step: 2’3’ cyclic nucleotide produced. His 12 is general base, His 119 is general acid
Transesterification
5'... O
5'... O
O Pyrimi dine
His 12
Nucleophilic attack
of 2' O on phosphate
His 119
HN
NH+
:N
NH+
O O
H
Base abstracts
O P O
proton from 2' OH
O
O
Bas e
Acid protonates
5' leaving group
Charge
A stabilization
Lys 41
O
O
Pyrimi dine
O O
P
O OHO
P
O
O
+H 3 N
O
O
5'... O
O
O
3'... O OH
O
Bas e
3'... O OH
Trigonal bipyramidal transition state
Bas e
3'... O OH
Intermediate
Second Step:
Hydrolysis of 2',3' cyclic phosphate intermediate
5'... O
O
Acid protonates
2' OH leaving group
5'... O
O Pyrimi dine
His 119
His 12
HN
Base abstracts
Pyrimi dine proton from H 2O
NH+
O O
P
-O O
H
O
His 12 is general acid, His 119 is general base
:N
H
NH+
Nucleophilic attack of
H 2 O on phosphate
O OH
O P O
O
3' phosphate
Proposed mechanism of RNase A catalysis. The unionized form of His 12 accepts a
proton from the 2' OH which enhances its nucleophilicity. The protonated form of His 119
begins to donate its proton to the 5' O, and the 2'O begins to form a bond with P to form a
pentacoordinate transition state. The negative charge that develops is stabilized
electrostatically by the nearby positively charged side chain of lysine 41. The bond between
P and the 5'-O breaks when the proton from histidine 119 is completely transferred. At the
same time, a bond between P and the 2'-O becomes fully formed, producing the 2',3'-cyclic
intermediate. Hydrolysis of the cyclic intermediate is a reversal of the first stage with H2O
replacing the 5'-O component that was removed. Histidine 12 is now the proton donor and
histidine 119 is the proton acceptor.
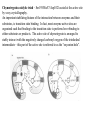
Geometry of the pentacovalent transition state. The central
phosphorus atom is transiently bonded to 5 oxygen atoms. Three
oxygens are coplanar with the phosphorus. The oxygen atoms of the
leaving group is at one apex, and the oxygen atom of the attacking group
is at the other apex of the trigonal bipyramid (in-line attack).
Evidence for RNase A mechanism
pH dependence of Vmax/KM for RNase A catalyzed
hydrolysis of cytidine-2',3'-cyclic phosphate. Bell shaped
curve suggests a catalytic role for functional groups with
pK's of 5.4 and 6.4, consistent with histidines.
Crystal structure of RNase A complex with cytidine 2'3'cyclic phosphate intermediate. Shows histidines and lysine
appropriately positioned in the active site. Note hydrogen
bonding interactions between cytosine and threonine 45
that confer substrate specificity.
Chemical modification. Iodoacetate alkylates histidine 119 or histidine 12 but not both in the
same molecule. Alkylation of either histidine eliminates catalysis. Complex formation with
substrate or competitive inhibitors protects histidines from modification.
B. Metal ion catalysis
1. Water ionization. A metal ion's charge makes its bound water molecules more acidic than
free H2O and therefore a source of OH- ions even below neutral pH (Metal ions have been
called "Super acids").
Mg 2+ H2O
Mg 2+ OH - + H +
pKa = 11
2. Charge shielding - metal ions can have charge > +1.
3. Oxidation-Reduction
The Hammerhead Catalytic RNA
The hammerhead ribozyme, like RNase A,
catalyzes a transesterification reaction to cleave
the phosphodiester backbone of substrate
RNAs yielding products with 5' hydroxyl and
2'3'cyclic phosphate termini. Unlike the RNase
A-catalyzed reaction, the hammerhead reaction
does not proceed through hydrolysis of the 2',3'
cyclic phosphate.
G C 5'
Substrate
GC
Ribozyme
A U
CG
cleavage site
A U
A
C
A
A
AGGAU
U GGCC G
U GCCGG
UCCUGGG5'
C
A
G AGU
U
Hammerhead Catalytic RNA
The hammerhead ribozyme obviously has no amino acid side chains to carry out proton
transfer and charge-shielding functions. RNAs are, however, capable of binding metal ions
with high specificity and affinity and the hammerhead ribozyme appears to make use of
metal ions to carry out both charge shielding and proton transfer functions.
C. Covalent catalysis - Transient formation of a catalyst-substrate covalent bond
-Provides an alternative reaction pathway, with two lower energy transition states
1. A nucleophile (electron-rich group with a strong tendency to donate electrons to an
electron-deficient nucleus) on the enzyme displaces a leaving group on the substrate,
forming a covalent bond.
2. The enzyme substrate bond decomposes to form product and free enzyme.
-Covalent catalyst must be a good nucleophile and a good leaving group - highly mobile
electrons (imidazole of His, thiol of Cys, carboxyl of Asp, hydroxyl of Ser).
Chymotrypsin, 25 kd serine protease, catalyzes hydrolysis of proteins in the small
intestine. Chymotrypsin catalyzes hydrolysis of esters as well as peptide bonds which has
been useful for analysis of the catalytic mechanism, although not physiologically relevant.
Model reaction in which hydrolysis of acyl-enzyme intermediate is slow
O
O
CH 3 C o
NO2 + Chymotrypsin
fast
p-Nitrophenylacetate
-O
NO2
p-Nitrophenylate
Formation of the acyl-enzyme intermediate
occurs during the initial rapid phase and
slower hydrolysis (deacylation) of the acylenzyme intermediate occurs during the
second, slower phase.
chymotrypsin
+
CH 3 C
Acyl-enzyme intermediate
slow
[p-Nitrophenylate], M
24M
20
16M
10
8M
2
4
6
8
Time (min)
10
12
H 2O
H+
O
CH 3 C
O-
Acetate
32M
30
chymotrypsin
The plot at left shows the concentration of
p-nitrophenol produced as a function of time in
reactions containing different concentrations
of chymotrypsin and a large excess of
p-nirophenylacetate. An initial rapid phase
("burst") is followed by a slower phase. The
size of the initial burst is proportional to the
enzyme concentration. "Burst" kinetics
provide evidence for a stable, enzyme-linked
intermediate.
+ chymotrypsin
First stage in peptide bond hydrolysis: acylation. Hydrolysis of the peptide bond starts with an
attack by the oxygen atom of the Ser195 hydroxyl group on the carbonyl carbon atom of the
susceptible bond. The carbon-oxygen bond of this carbonyl group becomes a single bond, and
the oxygen atom acquires a net negative charge. The four atoms now bonded to the carbonyl
carbon are arranged as a tetrahedron. Transfer of a proton from Ser195 to His57 is facilitated
by Asp102 which (i) precisely orients the imidazole ring of His57 and (ii) partly neutralizes the
positive charge that develops on His57 during the transition state. The proton held by the
protonated form of His57 is then donated to the nitrogen atom of the peptide bond that is
cleaved. At this stage, the amine component is hydrogen bonded to His57, and the acid
component of the substrate is esterified to Ser195. The amine component diffuses away.
Oxyanion
hole
Second stage in peptide hydrolysis: deacylation. The acyl-enzyme intermediate
is hydrolyzed by water. Deacylation is essentially the reverse of acylation with
water playing the role as the attacking nucleophile, similar to Ser195 in the first
step. First, a proton is drawn away from water. The resulting OH- attacks the
carbonyl carbon of the acyl group that is attached to Ser195. As in acylation, a
transient tetrahedral intermediate is formed. His57 then donates a proton to the
oxygen atom of Ser195, which then releases the acid component of the substrate,
completing the reaction.
Oxyanion
hole
Chymotrypsin catalytic triad – Ser195/His57/Asp102 located at the active site
by x-ray crystallography.
An important stabilizing feature of the interaction between enzymes and their
substrates, is transition state binding. In fact, most enzyme active sites are
organized such that binding to the transition state is preferred over binding to
either substrates or products. The active site of chymotrypsin is arranged to
stably interact with the negatively charged carbonyl oxygen of the tetrahedral
intermediate – this part of the active site is referred to as the “oxyanion hole”.
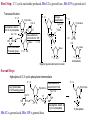
Mechanism of Protein Splicing:
The protein splicing pathway
consists of four nucleophilic
displacements. X represents the S or
O atom of the Cys/Ser/Thr
sidechains.
From: Perler, FB (1998) Cell 92, 1-4