* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download DENS 521 3rd S
Survey
Document related concepts
Human microbiota wikipedia , lookup
Marine microorganism wikipedia , lookup
Trimeric autotransporter adhesin wikipedia , lookup
Gastroenteritis wikipedia , lookup
Bacterial cell structure wikipedia , lookup
Clostridium difficile infection wikipedia , lookup
Neonatal infection wikipedia , lookup
Urinary tract infection wikipedia , lookup
Disinfectant wikipedia , lookup
Carbapenem-resistant enterobacteriaceae wikipedia , lookup
Anaerobic infection wikipedia , lookup
Traveler's diarrhea wikipedia , lookup
Bacterial morphological plasticity wikipedia , lookup
Staphylococcus aureus wikipedia , lookup
Transcript
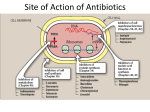
DENS 521 Clinical Dental Therapeutics 3rd Lecture By Abdelkader Ashour, Ph.D. Phone: 4677212 Email: [email protected] Resistance to b-lactams I. Production of b-lactamases Bacteria can destroy b-lactam antibiotics enzymatically by a group of enzymes called b- lactamases The production of b-lactamases is considered the principal cause of bacterial resistance to b-lactam antibiotics The introduction of new classes of b-lactams has invariably been followed by the emergence of new b-lactamases capable of degrading them, as an example of rapid bacterial evolution under a rapidly changing environment Some b-lactamases are relatively substrate specific, and these are described as either penicillinases or cephalosporinases Other "extended spectrum" enzymes are less discriminant and can hydrolyze a variety of b-lactam antibiotics Resistance to b-lactams I. Production of b-lactamases, contd. b-Lactamases are grouped into four classes: Class A b-lactamases include the extended-spectrum b-lactamases, which degrade penicillins, some cephalosporins and carbapenems Class B b-lactamases are Zn2+-dependent enzymes that destroy almost all b-lactams Class C b-lactamases are active against cephalosporins Class D includes cloxacillin-degrading enzymes. They also are active against some cephalosporins Class A and D enzymes are inhibited by the commercially available blactamase inhibitors, such as clavulanate and sulbactam Examples of bacteria that produce b-lactamases are staphylococcus aureus and many strains of H. influenzae, Neisseria and Pseudomonas Resistance to b-lactams II. The occurrence of PBPs with low affinity to cephalosporins The microorganism may be intrinsically resistant because of structural differences in the PBPs that are the targets of these drugs A sensitive strain may acquire resistance of this type by the development of high-molecular-weight PBPs that have decreased affinity for cephalosporins and penicillins, requiring clinically unattainable concentrations of the drug to effect its bactericidal activity Example 1: Methicillin-resistant S. aureus (MRSA) are resistant by means of acquisition of an additional high-molecular-weight PBP with a very low affinity for all b-lactam antibiotics Example 2: Cephalosporin resistance in Streptococcus pneumoniae is caused by altered PBPs (2 of the 5 high molecular weight PBPs) Resistance to b-lactams III. Decreased permeability to the drug Decreased penetration through the outer membrane prevents the drug from reaching the target PBP In G+ve bacteria, the peptidoglycan polymer is very near the cell surface, thus the small b-lactam antibiotic molecules can penetrate easily to the PBPs, where the final stages of the synthesis of the peptidoglycan take place G-ve organisms have an outer membrane that limits penetration of b-lactam antibiotics Some small hydrophilic antibiotics can diffuse through aqueous channels in the outer membrane that are formed by proteins called porins An extreme example is P. aeruginosa, which is intrinsically resistant to a wide variety of antibiotics because it lacks the classical high-permeability porins Active efflux pump serves as another mechanism of resistance, removing the antibiotic from its site of action before it can act This is an important mechanism of b-lactam resistance in P. aeruginosa, E. coli and Neisseria gonorrhoeae Examples of Penicillins Classification of Penicillins, On the Basis of Antibacterial Spectrum I. Narrow-spectrum: 1. Natural Penicillins Examples: benzylpenicillin (penicillin G), phenoxymethylpenicillin (penicillin V) Active against: most gram-positive bacteria with the exception of penicillinase-producing S. aureus most Neisseria species and some gram-negative anaerobes Not active against: most gram-negative aerobic organisms Penicillin G is the dug of choice for infections due to Neisseria meningitidis, Bacillus anthracis, Clostridium perfringens and tetani, Corynebacterium diphtheriae and Treponema pallidum….. Penicillin V is less active than penicillin G against Neisseria species. It is satisfactory substitute for penicillin G against Streptococcus pneumonia and S. pyogenes. It is the first choice in the treatment of odontogenic infections Tetracycline, a bacteriostatic antibiotic, may antagonize the bactericidal effect of penicillin, and concurrent use of these drugs should be avoided Adverse effects are generally uncommon The most important adverse effects are due to hypersensitivity with manifestations ranging from skin eruptions to anaphylactic shock Classification of Penicillins, On the Basis of Antibacterial Spectrum I. Narrow-spectrum Penicillins: 1. Natural Penicillins, contd. Pharmacokinetics: Penicillin G diffuses widely, attaining therapeutic concentrations in most body tissues The t1/2 of penicillin G is less than 1 hour and it is eliminated primarily by renal tubular secretion. This secretion can be inhibited by probenecid (this would prolong serum penicillin levels) Because renal dysfunction will compromise the elimination of penicillin, dosages may need to be reduced in patients with renal insufficiency (esp. in severe cases) Procaine penicillin is best suited to the single-dose outpatient treatment of very sensitive organisms (e.g., penicillin-sensitive N. gonorrhea and group A streptococci) Benzathine penicillin is another long-acting preparation given IM. It is used for prophylaxis of rheumatic fever and for treatment of syphilis Penicillin V is a much more resistant to gastric acid than is penicillin G and therefore better absorbed from the GIT. It is the orally-active form of penicillin All oral penicillins are best given on an empty stomach to avoid the absorption delay caused by food Classification of Penicillins, On the Basis of Antibacterial Spectrum I. Narrow-spectrum, contd. 2. Beta-Lactamase Resistant Penicillins Examples: methicillin, dicloxacillin, flucloxacillin Antibacterial Activity: These penicillins are resistant to staphylococcal lactamases They are also active against other bacteria for which penicillin G is indicated, but they are much less active than penicillin G II. Broad Spectrum Penicillins Examples: aminopenicillins such as ampicillin and amoxicillin These drugs retain the antibacterial spectrum of penicillin and have improved activity against G-ve organisms They are destroyed by b-lactamases Ampicillin and amoxicillin are among the most useful antibiotics for treating children suffering from infections caused by sensitive G-ve aerobic bacteria, enterococci, and b-lactamase-negative H. influenzae Amoxicillin is the favored drug for the treatment of acute otitis media Plasma concentrations of amoxicillin are usually twice those of ampicillin after an equivalent oral dose. The distribution and excretion characteristics of these penicillins are similar to those of penicillin Classification of Penicillins, On the Basis of Antibacterial Spectrum 3. Anti-Pseudomonal Penicillins Examples: pipracillin, azlocillin and mezlocillin These antibiotics have a broader spectrum of G-ve activity than do the aminopenicillins, and include activity against most strains of P. aeruginosa These antibiotics are used in the treatment of urinary tract, lung and bloodstream infections caused by ampicillin-resistant enteric G-ve pathogens Beta-lactamase Inhibitors These drugs competitively inhibit b-lactamase enzymes, restoring the original spectrum of activity to enzyme-susceptible antibiotics Some infections are polymicrobial and may involve anaerobes; for these the addition of a b-lactamase inhibitor might be of value These infections include infected animal and human bites, odontogenic infections, chronic sinusitis and intra-abdominal infections b-lactamase inhibitors in clinical use include clavulanic acid (usually combined with amoxicillin Augmentin®), sulbactam (usually combined with ampicillin Unasyn®) and tazobactam (usually combined with piperacillin Zosyn®)