* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download savaysa
Survey
Document related concepts
Psychedelic therapy wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Neuropharmacology wikipedia , lookup
Discovery and development of direct Xa inhibitors wikipedia , lookup
Adherence (medicine) wikipedia , lookup
Prescription costs wikipedia , lookup
Drug interaction wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Theralizumab wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Discovery and development of direct thrombin inhibitors wikipedia , lookup
Transcript
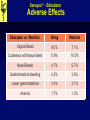
Savaysa™ - Edoxaban Manufacturer: Daiichi Sankyo FDA Approval Date: 01/08/2015 Savaysa™ - Edoxaban Clinical Application • Indications: • To reduce the risk of stroke and systemic embolism (SE) in patients with nonvalvular atrial fibrillation. • Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE) following 5 to 10 days of initial therapy with a parenteral anticoagulant. • Place in therapy: • Edoxban is the third factor Xa inhibitor approved for NVAF • Noninferior to warfarin, with significant lower risk of bleeding • Impaired renal function Savaysa™ - Edoxaban Clinical Application • Contraindications: • Active pathological bleeding • Black Box Warnings: • Reduced efficacy in nonvalvular atrial fibrillation patients with CrCl >95 mL/minute – Edoxaban should not be used in patients with CrCL >95 mL/minute. • Premature discontinuation of edoxaban increases the risk of ischemic events • Spinal/Epidural hematomas – Epidural or spinal hematomas may occur in patients treated with edoxaban who are receiving neuraxial anesthesia or undergoing spinal puncture. Savaysa™ - Edoxaban Clinical Application • Warnings / Precautions: • May increase the risk of bleeding; serious, potentially fatal bleeding may occur. • Not recommended for use in patients with mechanical heart valves or moderate to severe mitral stenosis; safety and efficacy have not been established. • Patients with DVT and/or PE and body weight ≤60 kg, reduce dose to 30mg • Discontinue edoxaban at least 24 hours prior to elective surgery or invasive procedures. Savaysa™ - Edoxaban Clinical Application • Pregnancy: • Category C • Lactation: • Excretion in breast milk unknown/not recommended. • Due to the potential for serious adverse reactions in the nursing infant, the decision to discontinue nursing or to discontinue the drug should be made taking into account the importance of treatment to the mother. Savaysa™ - Edoxaban Drug Facts • Pharmacology: • Edoxaban, a selective factor Xa inhibitor, inhibits free factor Xa and prothrombinase activity and inhibits thrombin-induced platelet aggregation. • Inhibition of factor Xa in the coagulation cascade reduces thrombin generation and thrombus formation. Savaysa™ - Edoxaban Drug Facts • Pharmacokinetics: A Bioavailability: 62% D Vd: 107 L with ~55% protein binding M Not significantly metabolized E Urine (primarily unchanged); renal clearance: ~50% of total clearance. T1/2: 10 to 14 hours Savaysa™ - Edoxaban Drug Interactions • Drug Interactions – Object Drugs: • Anticoagulants, Antiplatelets, and Thrombolytics – Co-administration may increase the risk of bleeding (anticoagulants, aspirin, other platelet aggregation inhibitors, and/or NSAIDs) Savaysa™ - Edoxaban Drug Interactions • Drug Interactions – Precipitant Drugs: • P-glycoprotein Inducers: ↓concentration of Edoxaban • Rifampin • P-glycoprotein Inhibitors: concentration of Edoxaban • Verapamil, quinidine or the short-term use of azithromycin, clarithromycin, erythromycin, oral itraconazole or oral ketoconazole. • NVAF: No dosage adjustment • DVT/PE: May need to dose reduce Edoxaban Savaysa™ - Edoxaban Adverse Effects Edoxaban vs. Warfarin: 60mg Warfarin Vaginal Bleed 9.0% 7.1% Cutaneous soft tissue bleed 5.9% 10.0% Nose Bleeds 4.7% 5.7% Gastrointestinal bleeding 4.2% 3.6% Lower gastrointestinal 3.4% 3.1% Anemia 1.7% 1.3% Savaysa™ - Edoxaban Monitoring Parameters • Efficacy Monitoring: • Routine monitoring of coagulation tests not required • Toxicity Monitoring: • Monitor for signs and symptoms of bleeding including neurological impairment. Savaysa™ - Edoxaban Prescription Information • Dosing: • DVT/PE: Oral: 60 mg once daily after 5 to 10 days of initial therapy with a parenteral anticoagulant. • Patient weight ≤60 kg: 30 mg once daily • Concomitant therapy with specific P-gp inhibitors: 30 mg once daily • Nonvalvular atrial fibrillation: Oral: 60 mg once daily • Cost: – Uptodate (03/01/2015) Brand – Generic Savaysa – edoxaban Dose $ 30 day 15 mg, 30mg, 60mg tablets (30) $333 Edoxaban versus Warfarin in Patients with Atrial Fibrillation SAVAYSA™ - EDOXABAN LITERATURE REVIEW Giugliano RP, et al. NEJM 2013; 369:2093-2104 Savaysa™ - Edoxaban Literature Review • Phase 3, randomized, double-blind, doubledummy, non-inferiority • Primary Endpoint: time to the first adjudicated stroke (ischemic or hemorrhagic) or systemic embolic event • Principal safety end point – adjudicated major bleeding • Secondary composite end points: stroke, systemic embolic event, or death from cardiovascular causes (including bleeding) • Treatment: 1:1:1 ratio, warfarin, high-dose (60mg) or low-dose (30mg) edoxaban Giugliano RP, et al. NEJM 2013; 369:2093-2104 Savaysa™ - Edoxaban Literature Review • Inclusion Criteria • Age ≥21 years old • History of AF of any duration within the prior 12 months • CHADS2 index score ≥2 • Exclusion Criteria • AF due to a reversible disorder • Severe renal dysfunction (CrCl < 30 mL/min) • Condition associated with a high risk of bleeding • Moderate or severe mitral stenosis Giugliano RP, et al. NEJM 2013; 369:2093-2104 Savaysa™ - Edoxaban Literature Review Warfarin (N=7036) High-Dose Edoxaban (N=7035) Low-Dose Edoxaban (N=7034) 72 72 72 37.5% 37.9% 38.8% ≤3 77.4% 77.1% 77.8% 4-6 22.6% 22.9% 22.2% CrCl ≤50 ml/min 19.3% 19.6% 19.0% Weight ≤60 kg 10.0% 9.7% 9.9% Use of verapamil or quinidine 3.5% 3.7% 3.7% Patient Characteristic (n=21,105) Age (yr) – Median Female CHADS2 score Dose reduction at randomization Giugliano RP, et al. NEJM 2013; 369:2093-2104 Savaysa™ - Edoxaban Literature Review • Results: (Median follow-up 2.8yrs) • The annualized rate of the primary end point of stroke or systemic embolism • 1.50% with warfarin • 1.18% with high-dose edoxaban (P<0.001 for noninferiority) • 1.61% with low-dose edoxaban (P=0.005 for noninferiority) • The annualized rate of major bleeding • 3.43% with warfarin • 2.75% with high-dose edoxaban (P<0.001) • 1.61% with low-dose edoxaban (P<0.001). Giugliano RP, et al. NEJM 2013; 369:2093-2104 Savaysa™ - Edoxaban Literature Review • Conclusion • Both once-daily regimens of edoxaban were noninferior to warfarin • Edoxaban was associated with significantly lower rates of bleeding and death from cardiovascular causes. Giugliano RP, et al. NEJM 2013; 369:2093-2104 Savaysa™ - Edoxaban Summary • Savaysa™ (Edoxaban) is a factor Xa inhibitor, indicated to prevent stroke and systemic embolism in non-valvular atrial fibrillation and to treat DVT and PE. When used in the treatment of DVT/PE edoxaban requires 5 to 10 days of initial therapy with a parenteral anticoagulant. • Edoxaban is typically given as a once daily 60mg dose, but should be administered at lower doses for patients with impaired renal or hepatic function or if the patient weights ≤60 kg. • Edoxaban should not be used in NVAF patients with CrCL >95 mL/minute. • The most common side effects of edoxaban include; vaginal, cutaneous soft tissue, nose, and GI bleeding. Savaysa™ - Edoxaban References 1. Savaysa package insert. Daiichi Sankyo. January 2015. 2. Edoxaban: Drug information. Lexicomp Drug Information. Accessed through UpToDate on March 2, 2015. 3. Giugliano RP, et al. NEJM 2013; 369:2093-2104.