* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Drug Handling in kidney and liver disease 2005
Survey
Document related concepts
Orphan drug wikipedia , lookup
Drug discovery wikipedia , lookup
Theralizumab wikipedia , lookup
Wilson's disease wikipedia , lookup
Drug design wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Pharmacognosy wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Psychopharmacology wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Transcript
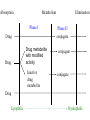
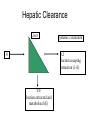
Drug Handling in kidney and liver disease Dr. Geoff Isbister Drug Action • Drugs tend to be small lipid-soluble molecules • Drugs must get access to sites of action • Drugs tend to bind to tissues, usually protein molecules • Drugs alter the actions of enzymes, ion channels and receptors Drug Action • ENZYME: example Angiotensin Converting enzyme inhibitors A I ----X---------->A II lowered A II -----> Reduced BP • ION CHANNELS: example Local Anesthetics Block Na channels--->Anesthesia • Receptor Binding – Receptors are specialised binding sites - often on cell surface- which have specificity for certain substances (incl drugs). Drugs may activate or block the receptor – Activation of the receptor changes the activity of the cell: eg adrenaline activates the beta 1 receptors in the heart and speeds up the heart – Drugs have selectivity for receptors: eg Histamine2 antagonists- reduce histamineinduced acid secretion and heal peptic ulcers Pharmacokinetics • The study of the action of the body on the drugs • Pharmacokinetics is the study of the time course of concentrations of drug in the body • The way the body handles drugs determines the dose, route and frequency of administration • The handling of drugs by the body can be split into absorption, distribution and elimination Pharmacokinetics • Rate of absorption determines the time to the peak concentration • The extent of absorption determines the height of the peak concentration and the AUC 30 25 20 15 10 5 0 0 1 5 9 13 Time after dosing Pharmacodynamics • The response of the tissue to the active free concentration of drug present at the site of action • May also be changed by disease processes Type of Disease • Renal disease – the nature of the disease doesn’t matter very much, the main determinant is the decline in GFR Routes of elimination - Kidney • Some drugs are water-soluble and are eliminated directly by the kidney – Molecules with MW below 20000 diffuse into glom filtrate. – examples: gentamicin, digoxin, atenolol – involves no chemical change to the drug – in most cases occurs by filtration (and depends on the GFR) – in a few cases (eg penicillin) some tubular secretion contributes to elimination • Highly lipid-soluble drugs are filtered into the tubules and then rapidly re-absorbed – High protein binding will reduce filtration Practical issues - treating real patients • Assessing kidney function is straightforward – serum creatinine reflects GFR – relationship between serum creatinine and GFR changes with age Effects of age on renal function • There is a steady and proportional decline in average GFR with increasing age • However the serum creatinine remains unchanged • Why is this? Effects of age on renal function (constant serum creatinine of 0.10 mmol/l) 100 90 80 70 60 50 40 30 20 10 0 20 40 60 80 Multiple Dosing - renally excreted drug Approx 5 half-lives to reach steady state 1 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 Elderly 0 12 24 36 48 60 72 84 96 Drug Types • Water soluble - excreted unchanged (by the kidney) • Lipid soluble - filtered but fully reabsorbed in the kidney - metabolised to polar products (filtered without reabsorption) A number of drugs are handled by tubular mechanisms • Two mechanisms – Active tubular secretion – important • Acidic drugs – frusemide, methotrexate, penicillins, salicylate, uric acid, probenecid • Bases – amiloride, morphine, quinine • Passive diffusion – After filtration lipid-soluble drugs will be re-absorbed passively. – Will depend on degree of ionization at certain pH levels Practical Examples of dosing in renal failure Gentamicin • Practice is changing - trend to once/daily dosing • The interval between doses may be >24 hours in the presence of renal failure and in the elderly • Toxicity relates to trough concentrations, particularly with prolonged therapy • Toxicity mainly affects the kidney and 8th cranial nerve Digoxin • In the presence of renal impairment the dose must be reduced • The dose is given once daily • Elderly people almost invariably have some renal impairment, so they usually require dose reduction - normally a halving of dose compared with young people Summary • Reduced elimination of drugs from the body in the elderly will lead to accumulation and toxicity • Disease and old age lead to reduced renal elimination of water-soluble drugs • Co-morbidity and concomitant drug therapy Hepatic Disease • Metabolism by the Liver : – role of metabolism – types of metabolism • Clearance – hepatic clearance • Liver disease Type of Disease • In liver disease the type of disease does matter: – Hepatitis – not much effect – Biliary obstruction – not much effect (initially) – Cirrhosis – has major effects on drug handling Assessing Function • Assessing liver function is hard - no single test of how well the liver metabolises drugs – Drug metabolism most likely to be impaired when the patient has cirrhosis, and has evidence of coagulation disturbances and low albumin Biotransformation • Majority produces metabolites that are : – less active – more polar and water soluble • Minority : – Pro-drugs that require metabolism to be active – active metabolites – more toxic (mutagenic, teratogenic etc.) Drugs with Active Metabolites DRUG ACTIVE METABOLITE allopurinol amitriptyline codeine diazepam procainamide prednisone primidone aspirin oxypurinol nortriptyline morphine oxazepam N-acetyl PA prednisolone phenobarbitone salicylate Types of Metabolism • Phase 1 Reactions – usually convert the parent drug into a more polar metabolite by introducing or unmasking a functional group (-OH, -NH2, -SH). Metabolite is usually inactive. • Phase 2 Reactions - Conjugation – an endogenous substrate (glucuronic acid, sulfuric acid, acetic acid, or amino acid) is attached to a functional group on the drug or phase I metabolite. Absorption Phase I Phase II conjugate Drug Drug Elimination Metabolism Drug metabolite with modified activity Inactive drug metabolite conjugate conjugate Drug Lipophilic Hydrophilic Phase I Reactions • Mixed Function Oxidase: – P450 enzyme system – induced and inhibited – hydroxylation and demethylation – family of isoenzymes • Monoamine Oxidase : catecholamines • Dehydrogenases :eg. Alcohol dehydrogenase Phase I - P450 System • • • • • FRAGILE High specificity Low volume Energy dependent First affected by liver disease Cytochrome P450 System • Not a single entity • Family of related isoenzymes (about 30) • Important for drug interactions : – Enzyme induction – Enzyme inhibition • Genetic polymorphism Phase II Reactions Conjugation • • • • • Glucuronidation Sulfation Acetylation Glutathione Glycine Phase II Reactions Conjugation • • • • • ROBUST High volume Low specificity Not energy dependent Less effected by liver disease Paracetamol toxicity – failure of Phase II Conjugation pathway saturates oxidation by P450 cytochrome pathway Formation of toxic metabolite NAPQI Initially detoxified by glutathione Glutathione depletion NAPQI accumulates and binds to tissue macromolecules - cell death Sites of Biotransformation • Liver • • • • Lung Kidney Large and small intestine Placenta Hepatic Clearance Liver Systemic circulation 0.2 fraction escaping extraction (1-E) 1.0 0.8 fraction extracted and metabolised (E) Extraction Ratio • High extraction ratio : – Effectively removed by the liver – Limited by hepatic blood flow – High first pass metabolism – Eg. Lignocaine, propranolol, diltiazem, morphine – Less effected by changes in intrinsic clearance, such as induction and inhibition Extraction Ratio • High Extraction ratio – Clearance approximates organ blood flow • Low Extraction ratio – Clearance proportional to free drug in the blood and intrinsic clearance of the liver Liver Disease • Severe disease before major effects on metabolism • Liver Disease : – Hepatocellular disease – Decrease liver perfusion • Type of metabolism : – Phase I – Phase II Disease Factors • Disease Type : – Acute hepatitis – little effect – Biliary Obstruction – little effect – Chronic Active Hepatitis – major effects – Cirrhosis – major effects • Indicators : – Established cirrhosis, varices, splenomegaly, jaundice, increased prothrombin time. Disease Factors • Poor perfursion • Cardiac failure : limits blood flow so effects those with high extraction ratios – Eg. Lignocaine – Combination with ischaemic liver injury • Other low perfusion states : – Other causes of shock Recent theories to account for impaired metabolism in cirrhosis • • • • Intact hepatocyte mass Sick cell theory Impaired drug uptake/shunting theory Oxygen limitation theory Type of Metabolism • Phase I, mainly P450 – Affected first • Phase II – Severe disease before any effect – Eg. Paracetamol poisoning. Other considerations • Renal function may be impaired in moderate to severe liver disease – Creatinine levels are not predictive • Pro-drug metabolism impairment – Eg ACE inhibitors • Pharmaco-dynamic disturbances – Tissues may be excessively sensitive to even low concentrations of the drug – eg morphone in the brain in the presence of severe liver disease