* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 13. Condensed azines. Quinoline. Isoquinoline. Acridine. Diazines
Peptide synthesis wikipedia , lookup
List of phenyltropanes wikipedia , lookup
Process chemistry wikipedia , lookup
Electrochemistry wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Transition state theory wikipedia , lookup
Citric acid cycle wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Stoichiometry wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Metalloprotein wikipedia , lookup
Butyric acid wikipedia , lookup
Drug discovery wikipedia , lookup
Organic chemistry wikipedia , lookup
Click chemistry wikipedia , lookup
Chemical reaction wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Biochemistry wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Biosynthesis wikipedia , lookup
Acid strength wikipedia , lookup
Petasis reaction wikipedia , lookup
Acid–base reaction wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
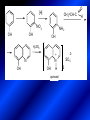
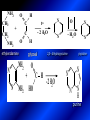
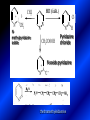
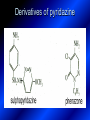
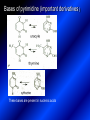
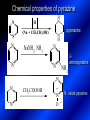
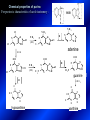
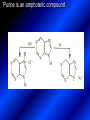
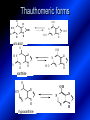
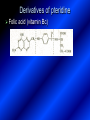
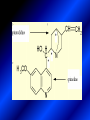
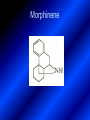
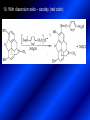
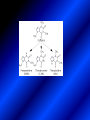
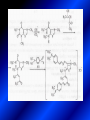
LECTURE № 13 THEME: Condensed azines. Quinoline. Isoquinoline. Acridine. Diazines. Purine. associate. prof. Ye. B. Dmukhalska, assistant. I.I. Medvid Plane 1. Receipt of quinoline and his derivatives. Synthesis of Skraupa and synthesis of Debner—Miller. Physical and chemical properties of quinoline. 2. Receipt of isoquinoline. Synthesis of Bischler-Napieralski. Physical and chemical properties of isoquinoline. 3. Structure, nomenclature, methods of getting and physical and chemical properties of acridine. 4. Methods of getting of sixmember heterocyclic connections with two heteroatoms 5. Structure, nomenclature, physical and chemical properties of pyridazine, pyrimidine, barbituric acid, pyrazine, purine, uric acid. 6. Structure, nomenclature and properties of azepines. Benzazepine, diazepine. Benzodiazepine, Oxazepam. Radedrol (nitrazepam). Seduxen (diazepam). 7. Classification of alkaloids. 1. Obtaining of quinoline and his derivatives. Synthesis of Skraup and synthesis of Debner—Miller. Physical and chemical properties of quinoline. The impotent condensed of sixmembered heterocycles connections are with one heteroatom is: quinoline isoquinoline acridine Quinoline, also known as 1-azanaphthalene, 1-benzazine, or benzo[b]pyridine, is a heterocyclic aromatic organic compound. It has the formula C9H7N and is a colourless hygroscopic liquid with a strong odour. Aged samples, if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents. Quinoline is mainly used as a building block to other specialty chemicals. Approximately 4 tonnes are produced annually according to a report published in 2005. Its principal use is as a precursor to 8-hydroxyquinoline, which is a versatile chelating agent and precursor to pesticides. Its 2- and 4-methyl derivatives are precursors to cyanine dyes. Oxidation of quinonline affords quinolinic acid (pyridine-2,3-dicarboxylic acid), a precursor to the herbicide sold under the name "Assert". The Skraup synthesis is a chemical reaction used to synthesize quinolines. It is named after the Czech chemist Zdenko Hans Skraup (1850-1910). In the archetypal Skraup, aniline is heated with sulphuric acid, glycerol, and an oxidizing agent, like nitrobenzene to yield quinoline. The Skraup synthesis place is taken in three stages. On the first stage glycerin is under the action of сoncentrated H2S04 to dehydration with formation of akrolein: On the second stage appearing akrolein enters into the reaction of condensation with an aniline: -H2O On the third stage of reaction of 1,2dihydroquinoline oxidizes nitrobenzol in to quinoline: Synthesis of Debner —Miller. The reaction is opened in 1881. On the first stage there is krothonic condensation of two molecules of aldehyde . Further there is cooperation of krothonic aldehyde with aniline. 2-methyl-1,2-dihydroquinoline 2-methyl-quinoline Chemical properties: 1. Reactions of heteroatom. quinoline chloride N-methylquinoline iodide N-acethylquinoline chloride 2. Reactions of electrophilic and nukleophilic substitutions . Reactions of electrophilic substitution in the molecula of quinoline is entered in position of 5 and 8. 6- quinoline sulphatic acid Reactions of nukleophilic substitution (is entered in position of 2) . 2- aminoquinoline 2- hydroxyquinoline 3. Reactions of reduction and oxidization. quinolinic acid Derivatives of quinoline 8-Hydroxyquinoline is an organic compound with the formula C9H7NO. It is a derivative of the heterocycle quinoline by placement of an OH group on carbon number 8. This colorless compound is widely used commercially, although under a variety of names. It is usually prepared from quinoline-8-sulfonic acid and from the Skraup synthesis from 2-aminophenol. NaOH -Na2SO3 8-Hydroxyquinoline is a monoprotic bidentate chelating agent. Related ligands are the Schiff bases derived from salicylaldehyde, such as salicylaldoxime and salen. The roots of the invasive plant Centaurea diffusa release 8-hydroxyquinoline, which has a negative effect on plants that have not co-evolved with it. The complexes as well as the heterocycle itself exhibit antiseptic, disinfectant, and pesticide properties. Its solution in alcohol are used as liquid bandages. It once was of interest as an anti-cancer drug. O [H] CH 2=CH-C NO 2 OH NH 2 OH OH H2SO4 2- + OH SO4 N N OH H quinozol 2 H 2. Receipt of isoquinoline. Synthesis of Bischler-Napieralski. Physical and chemical properties of isoquinoline. Isoquinoline, also known as benzo[c]pyridine or 2-benzanine, is a heterocyclic aromatic organic compound. It is a structural isomer of quinoline. Isoquinoline and quinoline are benzopyridines, which are composed of a benzene ring fused to a pyridine ring. In a broader sense, the term isoquinoline is used to make reference to isoquinoline derivatives. 1-Benzylisoquinoline is the structural backbone in naturally occurring alkaloids including papaverine and morphine. The isoquinoline ring in these natural compound derives from the aromatic amino acid tyrosine. Isoquinoline is a colourless hygroscopic liquid at room temperature with a penetrating, unpleasant odour. Impure samples can appear brownish, as is typical for nitrogen heterocycles. It crystallizes platelets that have a low solubility in water but dissolve well in ethanol, acetone, diethyl ether, carbon disulfide, and other common organic solvents. It is also soluble in dilute acids as the protonated derivative. In the Bischler-Napieralski reaction an β-phenylethylamine is acylated and cyclodehydrated by a Lewis acid, such as phosphoryl chloride or phosphorus pentoxide. The resulting 1-substituted-3,4-dihydroisoquinoline can then be dehydrogenated using palladium. The following BischlerNapieralski reaction produces papaverine. 1-substituted-3,4dihydroisoquinoline 1-substituted isoquinoline Chemical properties: 1. Reactions of electrophilic and nukleophilic substitutions . Reactions of electrophilic substitution in the molecula of isoquinoline is entered in position of 5 and 8. Reactions of nukleophilic substitutions tare place in position of 1. isoquinoline of chloride N-methylisoquinoline of iodide 2. Reaction of reduction 1,2,3,4tetrahydroisoquinoline 3. Reaction of oxidization 3,4- pyridinedicarbonic acid Methods of getting: 1. Condensation of diphenylamine with carbonic acids : diphenylamine acridine 2. Cyclization N-fenilanthranilic acid on the reaction of Drozdov—Mahidson—Hrihorovsky: N-fenilanthranilic acid anhidride chloride N-fenilanthranilic acid acridone-9 acridole-9 9,10-dihydroacridine 9-chloracridine acridine 1. Reactions of heteroatom. acridinium chloride N-oxide of acridine N-methylacridine iodide 2. Reactions of electrophilic and nukleophilic substitutions . 9-hydroxacridine, acridole-9 9- oxoacridine, acridone-9 3. Reactions of oxidization. acridinic acid 4. Reactions of reduction. 9,10-dehydroacridine, acridane Derivatives of acridine 9- Aminoacridine is an antiseptic and disinfectant. Acidylating flows on aminogroup: 9-N-acethylaminoacridine 9-aminoacridine chloride arcihine rivanol medicinal preparations COOH O2N Cl OC2H5 COOH H2N O2N NH O OH O2N OC2H5 POCl3 OC2H5 C POCl3 -HCl NH NH2 OC2H5 N N O2N Cl O2N OC2H5 OC2H5 NH3 -HCl [H] O2N N 9-amino-2-ethoxy-6-nitroacridine NH2 OC2H5 COOH + HC OH N H2N CH3 NH2 OC2H5 H2N N . COOH HC OH CH3 ethacridine lactate, rivanol, 6,9 – diamimo-2-ethoxyacridine lactate Sixmembered heterocycles connections are with two heteroatoms In addition to these three diazines, the bicyclic tetraaza compound, purine, is an important heterocyclic system. These ring systems, particularly that of pyrimidine, occur commonly in natural products. The pyrimidines, cytosine, thymine, and uracil are especially important because they are components of nucleic acids, as are the purine derivatives adenine and guanine. The рurine nucleus also occurs in such compounds as caffeine (coffee and tea) and theobromine (cacao beans). 4. Methods of getting of sixmember heterocyclic connections with two heteroatoms CH HC C H C H NH 2 NH 2 O O N - H 2O N maleinaldehide pyridazine O O H C CH OC H 2 H N 5 + 2 C OC H 2 C H ONa 2 5 2 C Malene ephir H N N C H O -C 2H 5O H H N O N 2 HO O H urea O 5 барбітурова barbituric acid Cl POCl 3 N 6H (Zn) N Cl N N Cl OH pyrimidine N OH These method use for obtaining pyridazine and his derivatives NH2 CH2 H O C to C - 2 H2O + CH2 O NH2 glyoxal NH2 NH2 N N - H2O N 2,3- dihydropyrazine pyrazine O + N O H ethylendiamine N N C H HO N N - 2 H2O N N H purine 5. Structure, classification, nomenclature, physical and chemical properties of pyridazine. Pyridazine is a heteroaromatic organic compound with the molecular formulaC4H4N2, sometimes called 1,2-diazine. It contains a six-membered ring with two adjacent nitrogen atoms. It is a colorless liquid with a boiling point of 208 °C. Pyridazine has no household use. It is mainly used in research and industry as building block for more complex compounds. The pyridazine structure is found within a number of herbicides such as credazine, pyridafol and pyridate. It is also found within the structure of several pharmaceutical drugs such as cefozopran, cadralazine, minaprine, hydralazine, and cilazapril. Pyridazine Other names 1,2-diazine, orthodiazine, oizine Properties Molecular formula C4H4N2 Molar mass 80.09 g mol−1 Appearance colorless liquid Density 1.107 g/cm3 Melting point -8°C Boiling point 208°C + N N I- CH3 HCl (í àäë.) CH3I Nmethylpyridazine iodide + N N N CH3COOOH + N Cl- N H Pyridazine chloride N-oxide pyridazine N _ O thethramethyleldiamine Derivatives of pyridazine 6. Structure, nomenclature, physical and chemical properties of pyrimidine. Three nucleobases found in nucleic acids (cytosine, thymine, and uracil) are pyrimidine derivatives: In DNA and RNA, these bases form hydrogen bonds with their complementary purines. Thus the purines adenine (A) and guanine (G) pair up with the pyrimidines thymine (T) and cytosine (C), respectively. Pyrimidine Properties Molecular formula C4H4N2 Molar mass 80.088 Melting point 20–22 °C Boiling point 123–124 °C + N N HCl H _ ê. HNO3 N Cl N NH2 NH2 N HO pyrimidine chloride N ê. H2SO4 N HO N N 4-amino-2-hydroxypyridine Br2 N N NH2 Br N NaNH2 N N NH2 N NO2 2- aminopyridine N N N NH2 Derivatives of pyrimidine Barbituric acid (2,4,6-trihydroxypyrimidine) Keto-enole and lactam-lactim tautomery 7. Structure, nomenclature, physical and chemical properties of barbituric acid. Barbituric acid or malonylurea or 4hydroxyuracil is an organic compound based on a pyrimidine heterocyclic skeleton. It is an odorless powder soluble in hot water. Barbituric acid is the parent compound of a large class of barbiturates that have central nervous system depressant properties, although barbituric acid itself is not pharmacologically active. The compound was discovered by the German chemist Adolf von Baeyer on 4. December 1864—the feast of St Barbara and therefore the name given to the compound—by combining urea and malonic acid in a condensation reaction. Malonic acid has since been replaced by diethyl malonate. Bases of pyrimidine (important derivatives ) These bases are present in nucleinic acids Vitamin B1 (thiamine) contain pyrimidine and thyazole ring connect through methyl group. Physiological active form of vitamin B1 in living organisms is cocarboxilaza, which take part in enzyme processes, in hydrocarbon exchange Orotic acid is primary compound in biosynthesis of pyrimidine bases Pyrazine Other names 1,4-Diazabenzene, p-Diazine, 1,4Diazine, Paradiazine, Piazine, UN 1325 Properties Molecular formula C4H4N2 Molar mass 80.09 g/mol Appearance White crystals Density 1.031 g/cm3 Melting point 52 °C Boiling point 115 °C Solubility in water Soluble The Gastaldi synthesis (1921) is another variation: Chemical properties of pyrazine N N N H N H pyperazine (Na + CH3CH2OH) NaNH2, NH3 N N H N 2aminopyrazine N NH2 N N N CH3COOOH + N _ O N- oxide pyrazine Purine Properties Molecular formula C5H4N4 Molar mass 120.112 Melting point 214 °C Synthesis of purine Traube method: condnsation 4,5diaminopyrimidines with carbonic acids The Traube purine synthesis (1900) is a classic reaction (named after Wilhelm Traube) between an amine substutited pyrimidine and formic acid Chemical properties of purine. For purine is characteristic of azole tautomery : NH Cl NH N N NH Cl Cl NH N (1:1) 2 Cl Cl adenine KOH N NH Cl Cl N OH OH OH N NH N NH N N N H N N 3 2 NH (1:1) 3 H 2N H N N N N Cl N NH H 2N NH N guanine H HNO O O N HN N N H hypoxanthine N HN O N H N H xanthine 2 Purine is an amphoteric compound Derivatives of purine Main derivatives of purine are oxopurines: Uric acid is colorless crystal compound, bad soluble in water, ethanol and ether, soluble in dilute base solutions and glycerin. Uric acid is dibases acid. Thauthomeric forms O HN N N O N H N H O OH H N HO OH N H N uric acid O OH N HN N H N H O N N HO N H N xanthine O OH N HN N N N N H hypoxanthine N N H Salts of uric acid called urats. Urats is bad soluble in water, except salts with litium (Li). In hydroxyform uric acid gives reactions of nucleophilic substitutions. Chemical properties O H O N H N O 2NaOH O N H H N N H + NaO HO N N H disodium salt of uric acid Cl N OH N _ + ONa _ OH N H N N H POCl3 N N Cl Cl N NH 2,6,8-threechlorpurine Reactions of oxidation aloxane urea alantoine Murexidne’s reaction is the qualitative reaction on uric acid By heating uric acid with nitrate acid and next adding of ammonium observe purpur-violet color purpure acid (enole form) murexide Reactions of reduction xanthine Hypoxantine and xantine have the same chemical properties as uric acid N-methyl derivatives of hypoxantine and xantine widely used in pharmacy Aminopurines aminoderivatives of purine – adenine and guanine present in nucleinic acids as purine’s bases. Maine Phenothiazine Phenothiazine (dibenzo-1,4-thiazine) – colorless crystal compound, insoluble in water, diethyl ether, well soluble in hot ethanol. Method of getting Chemical properties 1. Alkylation and acylation 2. Oxidation 3. Reaction of electrophilic sybstitution go in location 3 and 7 with oxidation of sulphur Derivatives of phenothiazine Pteridine (pyrazino[2,3-d]pyrimidine) Method of getting: condensation of 4,5diaminopyrimidins with 1,2-dicarbonile compounds Pteridine is light yellow crystal compound, soluble in water, ethanol, less soluble in diethyl ether and benzole. Pteridine is stable to oxidation, by acting of acids and bases pteridine cycle decompose. Gives reaction of electrophilic substitution, protonate on nitrogen atom in 1 location. Derivatives of pteridine Folic acid (vitamin Bc) Aloxasine and isoaloxasine These compounds include benzyl, pyrazine and hydrate pyriidine cycles Flavine is a primery compound of riboflavin: 11. Structure, nomenclature and properties of azepines. Benzazepine. Sevenmember heterocyclic ring compounds have received much attention in the past few years owing to its wide range of biological activity. Azepines are heterocycles of seven atoms, with a nitrogen replacing a carbon at one position. A well known azepine is caprolactam Skeletal formula of caprolactam. Sevenmember heterocycles which containing nitrogen Benzazepine: bicyclic structure consisting of fused benzene and azepine rings; many compounds with this structure react with biogenicamine receptors, and so are psychotropic and neurotropic. Examples of benzazepine include fenoldopam and galantamine. fenoldopam galantamine 12. Structure, nomenclature and properties of diazepine. Benzodiazepine. Diazepine is a sevenmember heterocyclic compound with two nitrogen atoms (e.g., in ring positions 1 and 2) and three double bonds. When diazepine combined with a benzene ring, these is the basis of the benzodiazepine family . In these compounds the nitrogen atoms are at the 1 and 4 positions as, for example, in clobazam (depending on the position of the fused benzene ring, the nitrogen atoms are also in positions number 1 and 4). 5-Phenyl-1,3-dihydro-2H-1,4benzodiazepin-2-on forms the skeleton on many of the most common benzodiazepine pharmaceuticals, such as diazepam (chloro-substituted). The benzodiazepines are a class of psychoactive drugs with varying hypnotic, sedative, anxiolytic (anti-anxiety), anticonvulsant, muscle relaxant and amnesic properties, which are mediated by slowing down the central nervous system. Benzodiazepines are useful in treating anxiety, insomnia, agitation, seizures, and muscle spasms, as well as alcohol withdrawal. They can also be used before certain medical procedures such as endoscopies or dental work where tension and anxiety are present, and prior to some unpleasant medical procedures in order to induce sedation and amnesia for the procedure. Benzodiazepines are also used to treat the panic that can be caused by hallucinogen intoxication. Benzodiazepines can cause a physical dependence and a benzodiazepine addiction to develop and upon cessation of long term use a benzodiazepine withdrawal syndrome can occur. 13. Oxazepam. Radedrol (nitrazepam). Seduxen O (diazepam). N Oxazepam (marketed in English speaking N countries under the following brand names Alepam, Medopam, Murelax, Noripam, Ox-Pam, Purata, Serax and Serepax), is a drug which is a benzodiazepine derivative. Oxazepam Oxazepam has moderate amnesic, anxiolytic, anticonvulsant, hypnotic, 7-clorine-3-hydroxysedative and skeletal muscle relaxant 5-phenil-1,3-dihydro2H-1,4-benzodiazepin-2-on properties compared to other benzodiazepines. OH Cl Oxazepam is an intermediate acting benzodiazepine. Oxazepam acts as inhibitor on the central nervous system. The halflife of oxazepam is 4-15 hours. Oxazepam has been shown to suppress cortisol levels. H O N O 2N N Nitrazepam is a nitrobenzodiazepine It is a 1,4 benzodiazepine, with the chemical name 7-nitro-5-phenyl-1,3dihydro-2H-1,4- benzodiazepin-2-on. Nitrazepam Nitrazepam is a type of benzodiazepine drug and is marketed in English speaking countries under the following brand names Alodorm, Arem, Insoma, Mogadon, Nitrados, Nitrazadon, Ormodon, Paxadorm, Remnos and Somnite. It is a hypnotic drug with sedative and motor impairing properties, anxiolytic, anticonvulsant and skeletal muscle relaxant properties. It is long acting drug, has lipophilic and hepatometabolitic properties via oxidative pathways. It acts on benzodiazepine receptors in the brain which are associated with the GABA receptors (gammaaminobutyric acid). GABA is a major inhibitor neurotransmitter in the brain, involved in inducing sleepiness, muscular relaxation and control of anxiety and seizures, and slows down the central nervous system. H 3C O N N Cl O diazepam (4N-oxide 7-chlorine-1-methyl- Diazepam first marketed as Valium by Hoffmann-La Roche, is a benzodiazepine derivative drug. It possesses anxiolytic, anticonvulsant, hypnotic, sedative, skeletal muscle relaxant and amnestic properties. It is commonly used for treating anxiety, insomnia, seizures, muscle spasms, alcohol withdrawal and benzodiazepine withdrawal. 5-phenil-1,3-dihydro-2Н1,4-benzodiazepin-2-оne ) Diazepam occurs as solid white or yellow crystals and has a melting point of 131.5 to 134.5 °C. It is odorless, and has a slightly bitter taste. The British Pharmacopoeia lists diazepam as being very slightly soluble in water, soluble in alcohol and freely soluble in chloroform. The United States Pharmacopoeia lists diazepam as soluble 1:16 in ethyl alcohol, 1:2 in chloroform, 1:39 in ether, and practically insoluble in water. Qualitative reactions on benzodiazepines 1. With concentrated acids (H2SO4, HCl, HClO4) derivatives of benzodiazepines form color salts. 2. Heterocyclic nitrogen atom gives positive reaction with common alkaloids precipitate reagents. 3. Specific reaction on benzodiazepines derivatives is formation of green color after pyrolisis. 4. Formation of azodays after primary hydrolysis: Nozepam 5. Belshteine probe use for determination of halogens. 6. Noozepam by heating with conc. H2SO4 hydrolyzed with formation of formaldehyde, which forms violet color with fuxinsulfite acid. Pyridine and piperidine group: piperine, coniine, trigonelline, arecoline, arecaidine, guvacine, cytisine, lobeline, nicotine, anabasine, sparteine, pelletierine. N N H Pyrrolidine and pyrolisidine group: hygrine, cuscohygrine, platyphylline, nicotine. N N H Tropane group: atropine, cocaine, ecgonine, scopolamine, catuabine. N CH 3 Quinoline group: quinine, quinidine, dihydroquinine, dihydroquinidine, strychnine, brucine, veratrine, cevadine. N Isoquinoline group: opium alkaloids (papaverine, narcotine, narceine), sanguinarine, hydrastine, berberine, emetine, berbamine, oxyacanthine. N Phenanthrene alkaloids: opium alkaloids (morphine, codeine, thebaine) Phenethylamine group: mescaline, ephedrine, dopamine Indole group: N H Tryptamines: serotonin, bufotenine, psilocybin Ergolines (the ergot alkaloids): ergine, ergotamine, lysergic acid Beta-carbolines: harmine, harmaline, tetrahydroharmine Yohimbans: reserpine, yohimbine Vinca alkaloids: vinblastine, vincristine Kratom (Mitragyna speciosa) alkaloids: mitragynine, 7hydroxymitragynine Tabernanthe iboga alkaloids: ibogaine, voacangine, coronaridine Strychnos nux-vomica alkaloids: strychnine, brucine Purine group: N N H N N Xanthines: caffeine, theobromine, theophylline 15. Alkaloids group of pyridine and piperine (nicotine, anabasine, lobeline). CH3 Systematic (IUPAC) name Nicotine is an alkaloid found in the nightshade family of plants (Solanaceae) which constitutes approximately 0.6–3.0% of dry weight of tobacco, with biosynthesis taking place in the roots, and accumulating in the leaves. 3-[2’-(N-methylpyrrolidil)]pyridine It functions as an antiherbivore chemical with particular specificity to insects; therefore nicotine was widely used as an insecticide in the past, and currently nicotine analogs such as imidacloprid continue to be widely used. Anabasine is a pyridine alkaloid found in the Tree Tobacco (Nicotiana glauca) plant, a close relative of the common tobacco plant (Nicotiana tabacum). It is similar to nicotine. Its principal (historical) industrial use is as an insecticide. Anabasine is present in trace amounts in tobacco smoke, and can be used as an indicator of a person's exposure to tobacco smoke. β-(α’-pyperidile)pyridine Pharmacology Anabasine is a nicotinic acetylcholine receptor agonist. In high doses, it produces a depolarizing block of nerve transmission, which can cause symptoms similar to those of nicotine poisoning and, ultimately, death by asystole. In larger amounts it is thought to be teratogenic in swine. Lobeline is a natural alkaloid found in "Indian tobacco" (Lobelia inflata), "Devil's tobacco" (Lobelia tupa), "cardinal flower" (Lobelia cardinalis), "great lobelia" (Lobelia siphilitica), and Hippobroma CH3 longiflora. In its pure form it is a white amorphous powder which is freely Systematic (IUPAC) name soluble in water. Lobeline has been used L-(-)-2benzoilmethyle-6-(2’-hydroxy- as a smoking cessation aid, and may 2’-phenylethyl)-1-methylpyperidine have application in the treatment of other drug addictions such as addiction to amphetamines or cocaine. Lobeline has multiple mechanisms of action, acting as a VMAT2 ligand, which stimulates dopamine release to a moderate extent when administered alone, but reduces the dopamine release caused by methamphetamine. 16. Alkaloids group of quinoline (quinine). Quinine Systematic (IUPAC) name (R)-(6methoxyquinolin-4-yl)- (8’vinylquinuclidin-2’-yl)methanol Quinine is a natural white crystalline alkaloid having antipyretic (fever-reducing), antimalarial, analgesic (painkilling), and anti-inflammatory properties and a bitter taste. It is a stereoisomer of quinidine. Quinine was the first effective treatment for malaria caused by Plasmodium falciparum, appearing in therapeutics in the 17th century. Since then, many effective antimalarials have been introduced, although quinine is still used to treat the disease in certain critical situations. Quinine is available with a prescription in the United States. Quinine is also used to treat nocturnal leg cramps and arthritis. Chemical structure Quinine contains two major fused-ring systems: The aromatic quinoline and the bicyclic quinuclidine . Qualitative reaction on quinine Thaleyoquine test: emerald-green color – with conc. H2SO4 – blue fluorescence; – with sodium nitroprusside - yellow sediment. 17. Alkaloids of group of quinoline and phenanthreneisoquinoline (papaverine, morphine, codeine). Papaverine is an opium alkaloid used primarily in the treatment of visceral spasm, vasospasm (especially those involving the heart and the brain), and occasionally in the treatment of erectile dysfunction. While it is found in the opium poppy, papaverine differs in both structure and pharmacological action from the other opium alkaloids (opiates). Papaverine Systematic (IUPAC) name 1-(3’,4’-dimethoxybenzyl)6,7-dimethoxyisoquinoline Qualitative reactions on papaverine 1. With conc. H2SO4 by heating – violet color after heating. 2. With conc. HNO3 – yellow color, that after heating becomes orange. H3CO H3CO H3CO N N HNO3 H3CO N HNO3 H3CO 0 CH2 t C CH2 H3CO O2N NO2 H3CO OCH3 H3CO OCH3 жовте забарвлення Yellow 3. With bromine water – yellow precipitate. CH2 NO2 H3CO OCH3 оранжеве забарвлення Orange 4. With Erdman reagent (H2SO4 conc.+HNO3 conc.) – red color. 5. With Phrede reagent ((NH4)2MoO4+H2SO4 conc.) – violet color after heating. 6. With Mandelin reagent (NH4VO3+H2SO4 conc.) – blue-green color becomes blue. 7. With Marci reagent (HCOH+H2SO4 conc.) – at first forms red color, then yellow and at the end orange. By adding bromine water and ammonium appears violet precipitate, which dissolves in alcohols. H3CO OCH3 C H2 H3CO H3CO OCH3 OCH3 C H2 OCH3 N+ N+ H H C H2 2OCH3 SO4 Systematic (IUPAC) name 3,6-dihydroxy-N-methyl- 4,5epoxymorphinene-7 Morphine is a highly potent opiate analgesic drug, is the principal active agent in opium, and is considered to be the prototypical opioid. Morphine was in 1803 the first alkaloid isolated from a plant source. Like other opioids, e.g. oxycodone, hydromorphone, and diacetylmorphine (heroin), morphine acts directly on the central nervous system (CNS) to relieve pain, particularly at the synapses of the nucleus accumbens. Morphine has a high potential for addiction; tolerance and both physical and psychological dependence develop rapidly. Chemistry Chemical structure of morphine in correct 3D configuration. The benzylisoquinoline backbone is shown in blue. Morphine is a benzylisoquinoline alkaloid with two additional ring closures. Most of the licit morphine produced is used to make codeine by methylation. It is also a precursor for many drugs including heroin (diacetylmorphine), hydromorphone, and oxymorphone. Replacement of the N-methyl group of morphine with an N-phenylethyl group results in a product that is 18 times more powerful than morphine in its opiate agonist potency. Morphinene Qualitative reactions on morphine 1. With Marci reagent – purpur color quickly becomes blue-violet (distinctive reaction between morphine and codeine). 2. With ammonium – white crystal precipitate dissolves in NaOH. 3. With Phrede reagent – at first forms violet color, that changes to blue and by standing – to green. 4. With FeCl3 – blue color. 6. Oxidation reaction with K3[Fe(CN)6] and FeCl3 – blue color: 7. With Mandelin reagent – violet color. 8. With Erdmane reagent – intense red color: 9. With conc. HNO3 – red-orange complex compound: 10. With diazonium salts – azoday (red color). Obtaining derivatives of morphine Codeine Systematic (IUPAC) name 6-hydroxy-N-methyl-3-methoxyCodeine (INN) or methylmorphine 4,5-epoxymorphinen-7 is an opiate used for its analgesic, antitussive and antidiarrheal properties. It is by far the most widely used opiate in the world and probably the most commonly used drug overall according to numerous reports over the years by organizations such as the World Health Organization and its League of Nations predecessor agency and others. Qualitative reactions on codeine 1. With Marci reagent – blue-violet color. 2. Formation of apomorphine. After heating with conc. H2SO4 and FeCl3 appears blue color that becomes red after adding 1 drop of dilute HNO3. 3. With conc. HNO3 – red color becomes yellow. 4. With AgNO3 – orange precipitate Ag3PO4. 5. With Erdman reagent (H2SO4 conc.+HNO3 conc.) -blue color after heating. 6. With Phrede reagent ((NH4)2MoO4+H2SO4 conc.) - green color becomes blue. 7. With Mandelin reagent (NH4VO3+H2SO4 conc.) - green color becomes blue. 8. With sodium nithropruside– yellow sediment. Alkaloids group of purine (caffeine, theobromine, theophylline). 1,3,7trimethylxanthine, trimethylxanthine, Caffeine is a bitter, white crystalline xanthine alkaloid that acts as a psychoactive stimulant drug and a mild diuretic. Caffeine was discovered by a German chemist, Friedrich Ferdinand Runge, in 1819. He coined the term "kaffein", a chemical compound in coffee, which in English became caffeine. Caffeine present in sugh plants:Coffea arabica, Thea sinensis and Cola acuminata Thea sinensis Theobroma cacao Common reaction on caffeine, theobromine and theophylline – Murexyde reaction Theobromine Systematic (IUPAC) name 3,7-dimethyl2,3,6,7-tetrahydro-1H-purine-2,6-dione Specific reactions on theobromine – theobromine reacts with NaOH and CoCl2, appears evanescent violet color and separates gray-blue precipitate of cobalt salt. Theophylline in these conditions forms cobalt salt - white sediment with pink tinge: – with HgCl3 - white crystal precipitate; – sodium salt of theobromine reacts with AgNO3 – forms gelatins mass (silver salt): A chocolate bar and melted chocolate. Chocolate is made from the cacao bean, which is a natural source of theobromine. The mean theobromine concentrations in cocoa and carob products are: Item Mean theobromine content (mg/g) Cocoa 20.3 Cocoa cereals 0.695 Chocolate bakery products 1.47 Chocolate toppings 1.95 Cocoa beverages 2.66 Chocolate ice creams 0.621 Chocolate milks 0.226 Carob products 0-0.504 Theophylline Systematic (IUPAC) name 1,3dimethyl-7H-purine-2,6-dione Synthesis Theophylline can be prepared synthetically from dimethylurea and ethyl 2-cyanoacetate. Specific reactions on theophylline – theophylline forms with CoCl2 salt - white sediment with pink tinge (look at the previous slide); – with alkali solution of sodium nitropruside – green color dissolved in excess of acid; – with HgCl3 - white crystal precipitate; – sodium salt of theophylline reacts with AgNO3 – forms gelatins mass (silver salt): 19. Alkaloids group of tropane (atropine, scopolamine, cocaine). Atropine is a tropane alkaloid extracted from Systematic (IUPAC) name (8methyl-8-azabicyclo[3.2.1]oct3-yl) 3-hydroxy-2phenylpropanoate; tropinic ester of tropic acid deadly nightshade (Atropa belladonna), jimsonweed (Datura stramonium), mandrake (Mandragora officinarum) and other plants of the Solanaceae family. Atropine Derivatives of tropane Tropine Scopine Echonine Common reaction on tropane alkaloids – rection of Vitaly-Moren In a porcelain cup to atropine add conc. HNO3 and heat to dry state – forms yellow polinitrocompound, dissolve this compound in acetone, addo, 0,5M alcohol solution of KOH. Appears violet color disappears by standing. Qualitative reactions on atropine – with picric acid – yellow precipitate; – with Marci reagent – yellow color; – formation of benzaldehyde: Scopolamine Systematic (IUPAC) name (-)-(S)-3-hydroxy-2-phenyl-propionic acid (1R,2R,4S,7S,9S)9-methyl-3-oxa9-aza-tricyclo[3.3.1.02,4]non-7-yl ester; Scopinic ester of tropic acid Scopolamine, known by the names levo-duboisine and hyoscine, is a tropane alkaloid drug with muscarinic antagonist effects. It is obtained from plants of the Solanaceae family (nightshades), such as Datura Stramonium. It is among the secondary metabolites of these plants. Therefore, scopolamine is one of three main active components of belladonna and stramonium tinctures and powders used medicinally along with atropine and hyoscyamine. Scopolamine has anticholinergic properties and has legitimate medical applications in very small doses. An overdose can cause delirium, delusions, dangerous elevations of body temperature, stupor and death. Cocaine Systematic (IUPAC) name methyl (1R,2R,3S,5S)-3(benzoyloxy)-8-methyl-8azabicyclo[3.2.1] octane-2carboxylate; methylester of benzoilechonine The coca plant, Erythroxylon coca. Cocaine (benzoylmethyl ecgonine) is a crystalline tropane alkaloid that is obtained from the leaves of the coca plant. The name comes from "coca" in addition to the alkaloid suffix -ine, forming cocaine. It is both a stimulant of the central nervous system and an appetite suppressant. Specifically, it is a dopamine reuptake inhibitor, a norepinephrine reuptake inhibitor and a serotonin reuptake inhibitor. Its possession, cultivation, and distribution are illegal for non-medicinal and non-government sanctioned purposes in virtually all parts of the world. Although its free commercialization is illegal and has been severely penalized in virtually all countries. Qualitative reactions on cocaine – with KMnO4 – violet crystal precipitate: – heating with conc. H2SO4 (specific smell of methylbenzoate, by standing forms crystals of benzoic acid) Alkaloids group of indole (reserpine, strychnine). Systematic (IUPAC) name 11,17-dimethoxy-16carbmethoxy-18-(3’,4’,5’trimethoxybenzoyloxy)aloyohim-bane N H3CO N H OCH3 H3COOC O C OCH3 OCH3 O OCH3 Rauwolfia serpentina Aloyohimbane 6 9 10 8 A 11 12 B 13 5 7 1 C 2 N H 3 4 N 21 D 14 20 19 15 E Àë î é î õ³ì áàí 16 17 18 17 Strychnine 1 2 4 N 16 6 A 3 18 E B 15 7 14 N 9 10 O F D 8 5 19 20 13 C 21 G 12 11 * HNO3 O 22 23 Strychnos Nux Vomica Thank you for attention!