* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Tuberculosis
Survey
Document related concepts
Neglected tropical diseases wikipedia , lookup
Neonatal infection wikipedia , lookup
Traveler's diarrhea wikipedia , lookup
Hepatitis B wikipedia , lookup
Globalization and disease wikipedia , lookup
Schistosomiasis wikipedia , lookup
Infection control wikipedia , lookup
Childhood immunizations in the United States wikipedia , lookup
Hepatitis C wikipedia , lookup
Management of multiple sclerosis wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Transcript
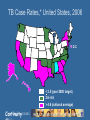
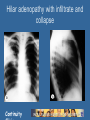
Tuberculosis Continuity Objectives • Know current epidemiologic trends in TB • Know indications for testing for TB exposure and the tests available • Be familiar with treatments for latent tuberculosis infections Continuity Background Epidemiology Continuity Cases Annually 99 million Cases Annually >1/3 in in India India and >1/3 and China China < 1 000 1 000 to 9 999 10 000 to 99 999 100 000 to 999 999 1 000 000 or more No Estimate Continuity Reported TB Cases* United States, 1982–2006 No. of Cases 28,000 26,000 24,000 22,000 20,000 18,000 16,000 14,000 12,000 10,000 1982 1986 1990 1994 Year Continuity 1998 2002 2006 TB Case Rates,* United States, 2006 D.C. < 3.5 (year 2000 target) 3.6–4.6 > 4.6 (national average) *Cases per 100,000. Continuity TB Case Rates by Age Group and Sex, United States, 2006 Cases per 100,000 12 10 8 6 4 2 0 <15 yrs 15–24 yrs 25–44 yrs Male Continuity 45–64 yrs Female >65 yrs Trends in TB Cases in Foreign-born Persons, United States, 1986–2006* No. of Cases Percentage 10,000 60 50 40 8,000 6,000 30 20 10 0 4,000 2,000 0 86 87 88 89 90 91 92 93 94 95 96 97 98 99 00 01 02 03 04 05 06 No. of Cases *Updated as of April 6, 2007. Continuity Percentage of Total Cases Drug Resistant TB Counted Cases defined † on Initial DST by Year, 1993–2006* *Reported incident cases as of 7/18/07 Case Count † Drug Susceptibility Test 12 10 8 6 4 2 Year of Diagnosis Continuity 2006 2005 2004 2003 2002 2001 2000 1999 1998 1997 1996 1995 1994 1993 0 TB in Children • WHO estimate of TB in children – 1.3 million annual cases – 450,000 deaths • 15% of TB in low-income countries children vs. 6% in United States Continuity MAKING THE DECISION TO TEST FOR TB The Initial “Test” for TB Infection is the History Continuity Who Should be Tested? • Those at epidemiological increased risk of having TB infection • Those at increased individual risk of developing TB disease if infected • ONLY test if you are going to treat the patient – a decision to test is a decision to treat Continuity Questionnaire Risk Assessment for TB Infection in Children - NYCDOH Ozuah et al. JAMA;285:451 Risk factor Sens. Contact to a case Birth/travel to endemic area Contact to HR adult Age > 11 yr Continuity 26 63 19 67 Spec. PPV NPV OR 99.6 89.7 96.6 71.0 38.9 5.4 4.9 2.1 99.3 99.6 99.2 99.6 92 15 7 5 Epidemiologically-Defined Groups with HIGH Prevalence of Tuberculosis Infection • Immigrants from areas of world with a high incidence of TB • Homeless persons, and other low income groups with poor access to health care • Elderly persons • Residents and employees in congregate living facilities serving persons at high risk of TB (correctional institutions, homeless shelters, health care facilities, nursing homes, assisted living facilities, AIDS housing) Continuity Underlying Medical Conditions Which Increase Risk for Progression to Active TB Disease – – – – – – – – – Continuity HIV infection Chronic renal failure Immunosuppressive Rx Diabetes mellitus Malignancy TNF Alpha blocker therapy Transplant recipients > 15 mg Prednisone/day Silicosis Incidence of Tuberculosis by Selected Risk Factors in Persons with a Positive TST Risk Factor TB Cases/1000 person-years Recent TB Infection Infection < 1 year past Infection 1-7 years past 12.9 1.6 HIV/AIDS 35.0-162 Injection Drug Use HIV-positive HIV-negative or unknown 76.0 10.0 Silicosis 68.0 Radiographic findings consistent with old TB 2.0-13.6 Weight Deviation from Standard (5% overweight 15% underweight) 0.7-2.6 Continuity HOW TO TEST Continuity Tuberculin Skin Testing Tuberculin Skin Testing Continuity Induration of >5mm Considered a Positive TST • HIV positive persons • Recent contacts of TB cases • Fibrotic Changes on CXR c/w old (not treated) TB • Patients with organ transplants or other immunosuppression • Prednisone therapy 15 mg/day > 1 month Continuity Induration of >10mm Considered a Positive TST • Recent arrivals (<5 yrs) high prevalence countries • Intravenous Drug Users • Residents/employees - high-risk congregate facilities (health care, prisons, shelters, etc.) Continuity Induration of >15mm Considered a Positive TST • TB lab personnel • Persons with “high-risk” medical conditions • Children <4 yrs or exposed to adults at risk Continuity Interferon Gamma Release Assays • Quantiferon – measure of interferon gamma in supernatant, currently at third generation test – Quantiferon Gold In-tube • Elispot – measure of individual T-cells that produce interferon gamma. Continuity Positive Skin Test Now what? Continuity Before Treatment of LTBI: Exclude Active Tuberculosis • • • • Absence of symptoms Negative CXR Negative medical evaluation Order and wait for sputum culture if any question Continuity Hilar adenopathy with infiltrate and collapse Continuity Miliary TB in a child Continuity Chest Radiograph “Pearls” • Hilar nodes, pleural disease – extrapulmonary, few bacteria • Cavitary disease – many bacteria • Parenchymal scars – NOT active, only needs preventive therapy (LTBI) IF scar is > 2.5 cm • Calcified node is functionally like a normal chest radiograph (very very few live AFB) Continuity Childhood TB diagnosed by: Combination of : Contact with infectious adult case Symptoms and signs Positive tuberculin skin test Suspicious CXR or CT/MRI Bacteriological confirmation Serology? Continuity Treatment Continuity Treatment of LTBI • Treatment regimens: – INH x 9 months – Alternative: Rifampin 600mg daily x 4 months for adults, 6 months for children and HIV+ – Possible: • INH & Rifampin x 3 to 4 months • INH, Rifampin, EMB & PZA x 2 months – No longer used: Rifampin/PZA x 2 months – New? Rifapentine & INH weekly x 12 weeks Continuity ISONIAZID PREVENTIVE THERAPY Worldwide Trials, 1955-1965 19 controlled trials in 11 countries: United States Japan Canada Netherlands Greenland France Mexico Tunisia Kenya India Philippines Over 100,000 participants Household contacts (6), Entire communities (3), Inactive pulmonary lesions (5), Children with primary TB (2), School children (1) Railway workers (1), Mentally ill patients (1) Continuity 25-92% protection How Much Isoniazid Is Needed for the Prevention of Tuberculosis? • Longer durations of therapy corresponded to lower TB rates among those who took 0-9 mo • No extra increase in protection among those who took >9 months Community based study, Bethel Alaska Comstock GW, 1999. Int J Tuberc. Lung Dis 3:847-850 Continuity IUATLD Study of INH Therapy for LTBI • Reduction in culture positive TB at 5 years all participants – 6 months therapy – 12 months therapy 65% 75% • Reduction in culture positive TB at 5 years in the group of completer-compliers (took > 80% of doses): – 6 months therapy – 12 months therapy Continuity 69% 93% Contacts Of INH Resistant TB • Four month regimen daily Rifampin for adults • Six month regimen daily Rifampin for HIV infected • Six month regimen daily Rifampin for children Continuity Treatment of Latent TB Infection in Special Situations • For children and adolescents (<18 years old): - Isoniazid for 9 months • For pregnant women: - Isoniazid for 9 or 6 months - may defer except for HIV- infected women and those recently infected with Mycobacterium tuberculosis • For persons exposed to isoniazid resistant TB: - Rifampin for 4 months • For persons likely infected with multidrug-resistant TB: - Pyrazinamide and ethambutol, or pyrazinamide and quinolone for 6-12 months (i.e., at least 2 drugs to which the organism is susceptible) Continuity TB and BCG Vaccination • Efficacy for adult pulmonary TB 0-80% in randomized clinical trials • Best efficacy against serious childhood disease – 64% protection against TB meningitis – 78% protection effect against disseminated TB • BCG important for young children, inadequate as single strategy Continuity Colditz GA et al. JAMA 1994; 271: 698-702.