* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Patent-Extenders
Survey
Document related concepts
Pharmaceutical marketing wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Psychopharmacology wikipedia , lookup
Orphan drug wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Compounding wikipedia , lookup
Neuropharmacology wikipedia , lookup
List of off-label promotion pharmaceutical settlements wikipedia , lookup
Drug design wikipedia , lookup
Pharmacognosy wikipedia , lookup
Drug interaction wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Theralizumab wikipedia , lookup
Transcript
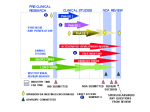
Patent-Extender Drugs: Loop-holes in the Law Sandy H. Yoo 4/14/06 “Patent-extender” Drugs An increasingly widespread tactic used by pharmaceutical companies to extend the life of their drug patents by creating a slightly altered version of the original drug, which is then eligible under FDA rules for at least another few years of market monopoly. Novak V. Bristol-Myers Squibb lobbies for a patent loop-hole. Time Magazine. Nov 2001 Overview of FDA approval process Pre-Clinical testing on animals Investigational New Drug (IND) Application Clinical Studies Phase I Phase II Phase III New Drug Application (NDA) NDA review for approval or non-approval Division Director “sign-off” on new drug Center for Drug Evaluation and Research (CDER) CDER is FDA’s branch responsible for approving new prescription drugs and generic drugs They oversee approval of IND, NDA, and review and give final approval or disapproval of drugs Pre-clinical Trials Manufacturers test their new drug on animals to ask: Is my drug safe to test in humans? Is there enough evidence of its pharmacological action to justify further clinical studies in humans? If the answers are YES, they submit an IND for approval to test in humans. Clinical Studies: Phase I Initial introduction of new investigational drug into humans Determine drug’s action, properties, and side-effects in humans Usually conducted in healthy human volunteers Clinical Studies: Phase II Early controlled clinical studies to obtain effectiveness of the drug for a particular indication Usually well-controlled, closely monitored Conducted in a relatively small number of patients (several hundred people) Clinical Studies: Phase III Expanded controlled and uncontrolled trials to evaluate risk-benefit ratio of drug Involves several hundred to thousands of people Gives basis for extrapolating data results to general population At any of the 3 phases, FDA can place a clinical hold New Drug Application & Review Data from pre-clinical trials and Phases I, II, III studies become part of the NDA NDA undergoes formal review by CDER The drug can be legally marketed once division director “signs-off” on new drug New Drug Development Process Generic Drugs Approval Process Generics use “abbreviated” new drug application (ANDA) Do not have to include preclinical and clinical data to establish safety and effectiveness Instead must prove BIOEQUIVALENCE If all requirements are met, generic is approved Hatch-Waxman Act 1984 Company can seek approval to market a generic before expiration of a brandname patent “Abbreviated” new drug application (ANDA) 1st company to submit ANDA gets 180exclusivity Patent holder may file infringement suit This delays approval of ANDA for 30months NDA’s Drug Classifications 1. New Molecular Entity 2. New Salt New Formulation New Combination of Two or More Drugs Duplication of Product (i.e., new manufacturer) New Indication (claim) (includes switch from prescription to OTC) 3. 4. 5. 6. 7. Already Marketed Drug Product - No Previously Approved NDA To name a few… Original Drug Ambien Glucophage Cipro Prozac Paxil Celexa Prilosec Wellbutrin Symbyax Claritin Later Drug Ambien CR Glucophage XR, Glucovance CiproXR, Proquin Serafem Paxil CR Lexapro Nexium Zyban Olanzapine/Fluoxetine Clarinex Manufacturer Sanofi Bristol-Myers Squibb Bayer-Schering Eli Lily Glaxo Smith Kline Forest AstraZeneca Glaxo Smith Kline Eli Lily Schering References 1. 2. 3. US FDA Center for Drug Evaluation and Research. Available at: http://www.fda.gov/cder/ Novak V. Bristol-Myers Squibb lobbies for a patent loophole. Time 2001, Nov 2. Field A. Doctoring the Hatch-Waxman Act. Stanford Graduate School of Business Research Paper.