* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download chapter 8 part 2
Woodward–Hoffmann rules wikipedia , lookup
George S. Hammond wikipedia , lookup
Homoaromaticity wikipedia , lookup
Asymmetric induction wikipedia , lookup
Elias James Corey wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Polythiophene wikipedia , lookup
Ene reaction wikipedia , lookup
Aromatization wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Stille reaction wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Discodermolide wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
Hydroformylation wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
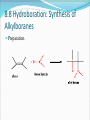
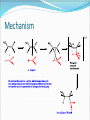
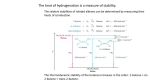
Alkenes and Alkynes II Addition Reactions Part 2 8.6 Alcohols From Alkenes through Oxymercuration-Demeruration: Markovnikov Addition Two steps mechanism that is useful to avoid rearrangement Both reaction take place very rapidly at RT or below Regioselectivity of OxymercurationDemercuration The orientation of the addition of the elements of water, H– and –OH, is in accordance with Markovnikov’s rule Examples Mechanism of Oxymercuration Mechanism Mechanism example Starting with an appropriate alkene, show all steps in the synthesis of 2-methyl-2-propanol Example Consider the following reaction Outline a likely mechanism for the solvomercuration step of this ether synthesis Show how you would use solvomercuration-demercuration to prepare tert-butyl methyl ether Why would one use Hg(OCCF3)2 instead of Hg(Oac)2 Answer for c The electron-withdrawing fluorine atoms in mercurc trifluoroacetate enhance the electrophilicity of the cation. Experiments have demonstrated that for the preparation of tertiary alcohols in satisfactory yields, the trifluoroacetate must be used rather than the acetate 8.7 Alcohols from Alkenes through Hydrocarboration-Oxidation: Anti-Markovnikov Syn Hydration Addition of water is indirect and two reactions are involved 1. addition of a boron atom and hydrogen to C= C Hydrolysis of the alkylborane intermediate to an alcohol and boric acid 8.8 Hydroboration: Synthesis of Alkylboranes Preparation Mechanism In each addition step, the boron atom becomes attached to the less subsituted carbon atom of the double bond A hydrogen atom is transferred from the boron atom to the other carbon atom of the double bond Con’t As this transition state is approached, electrons shift in the direction of the boron and away from the more substituted carbon atom of the double bond The more substituted carbon bears an electron-releasing alkyl group, it is better able to accommodate this positive charge Mechanism Example Starting with an appropriate alken, show the synthesis of tributyl borane 8.9 Oxidation and Hydrolysis of Alkylboranes These reactions are occurred in the same vessel by the addition of hydrogen peroxide in an aqueous base mechanism mechanism Example Starting with the appropriate alkene, show how you could use hydroboration-oxidation to prepare each of the alcohol 1-pentanol 2-methyl-1-pentenol