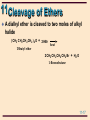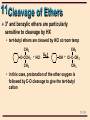* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download OC 2/e Ch 11
Woodward–Hoffmann rules wikipedia , lookup
Marcus theory wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Stille reaction wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Asymmetric induction wikipedia , lookup
Ene reaction wikipedia , lookup
Discodermolide wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Petasis reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
George S. Hammond wikipedia , lookup
Elias James Corey wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
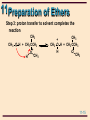
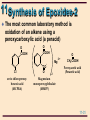
11 Organic Chemistry William H. Brown & Christopher S. Foote 11-1 11 Ethers & Epoxides Chapter 11 11-2 11 Structure The functional group of an ether is an oxygen atom bonded to two carbon atoms Oxygen is sp3 hybridized with bond angles of approximately 109.5°. • in dimethyl ether, the C-O-C bond angle is 110.3° H H •• H C H O •• C H H 11-3 11 Nomenclature: ethers IUPAC: the longest carbon chain is the parent. • name the OR group as an alkoxy substituent Common names: name the groups attached to oxygen followed by the word ether OH CH3 OCCH3 CH3 CH2 OCH 2 CH3 OCH2 CH3 Ethoxyethane (Diethyl ether) CH3 trans-2-Ethoxycyclohexanol CH3 2-Methoxy-2methylpropane (tert-Butyl methyl ether) 11-4 11 Nomenclature: ethers Although cyclic ethers have IUPAC names, their common names are more widely used • IUPAC: prefix ox- shows oxygen in the ring. The suffixes -irane, -etane, -olane, and -ane show three, four, five, and six atoms in a saturated ring. 2 3 1O Ethylene oxide 3 4 2 1O 4 4 3 5 2 1O Tetrahydrofuran Tetrahydropyran (THF) O 3 5 2 6 1O 1,4-Dioxane 11-5 11 Physical Properties Ethers are polar molecules • oxygen bears a partial negative charge • each carbon a partial positive charge 11-6 11 Physical Properties Ethers are polar molecules but, because of steric hindrance, only weak dipole-dipole attractive forces exist between their molecules in the pure liquid state Boiling points of ethers are • lower than alcohols of comparable MW • close to those of hydrocarbons of comparable MW Ethers hydrogen bond with H2O and are more soluble in H2O than are hydrocarbons 11-7 11 Preparation of Ethers Williamson ether synthesis: SN2 displacement of halide, tosylate, or mesylate by alkoxide ion CH3 - CH3 CHO N a Sodium isopropoxide + + CH3 I SN 2 Iodomethane (Methyl iodide) CH3 CH3 CHOCH3 + + - Na I 2-Methoxypropane (Isopropyl methyl ether) 11-8 11 Preparation of Ethers Williamson ether synthesis: yields are • highest with methyl and 1° halides, • lower with 2° halides (competing -elimination) • reaction fails with 3° halides (-elimination only) CH3 CH3 CBr - + CH3 O N a CH3 2-Bromo-2methylpropane + Sodium methoxide E2 CH3 + CH3 C= CH2 + CH3 OH + N a Br 2-Methylpropene - 11-9 11 Preparation of Ethers Acid-catalyzed dehydration of alcohols • diethyl ether and several other ethers are made on an industrial scale this way • a specific example of an SN2 reaction in which a poor leaving group is converted to a better one 2 CH3 CH2 OH Ethanol H2 SO 4 140°C CH3 CH2 OCH 2 CH3 + H2 O Diethyl ether 11-10 11 Preparation of Ethers • Step 1: proton transfer gives an oxonium ion : proton O transfer CH3 CH2 -O- H + H- O-S- O-H O O + CH3 CH2 -O- H + - :O-S- O-H H O An oxonium ion • Step 2: nucleophilic displacement of H2O by the OH group of the alcohol gives a new oxonium ion : + CH3 CH2 -O- H + CH3 CH2 -O- H H SN 2 + CH3 CH2 -O- CH2 CH3 + : O-H H A new oxonium ion H 11-11 11 Preparation of Ethers Step 3: proton transfer to solvent completes the reaction proton + transfer : + CH3 CH2 -O- CH2 CH3 O-H H H + : CH3 CH2 -O- CH2 CH3 + H O-H H 11-12 11 Preparation of Ethers Acid-catalyzed addition of alcohols to alkenes: yields are highest using • an alkene that can form a stable carbocation • methanol or a 1° alcohol CH3 CH3 C= CH2 + CH3 OH acid catalyst CH3 CH3 COCH3 CH3 2-Methoxy-2-methyl propane 11-13 11 Preparation of Ethers • Step 1: protonation of the alkene gives a carbocation CH3 CH3 C= CH2 + + H O CH3 H CH3 CH3 CCH3 + :O CH3 + H • Step 2: reaction of the alcohol (a Lewis base) with the carbocation (a Lewis acid) CH3 CH3 : CH3 CCH3 + HOCH3 + CH3 CCH3 + O CH3 H 11-14 11 Preparation of Ethers Step 3: proton transfer to solvent completes the reaction CH3 : CH3 O H + CH3 CCH3 + O CH3 H CH3 CH3 + O H + CH3 CCH3 :O H CH3 11-15 11 Cleavage of Ethers Ethers are cleaved by HX to an alcohol and an alkyl halide R-O-R + H-X R-O-H + R-X • cleavage requires both a strong acid and a good nucleophile; therefore, the use of concentrated HI (57%) and HBr (48%) • cleavage by concentrated HCl (38%) is less effective, primarily because Cl- is a weaker nucleophile in water than either I- or Br- 11-16 11 Cleavage of Ethers A dialkyl ether is cleaved to two moles of alkyl halide ( CH3 CH2 CH 2 CH 2 ) 2 O + 2 HBr Dibutyl ether heat 2 CH3 CH2 CH2 CH2 Br + H2 O 1-Bromobutane 11-17 11 Cleavage of Ethers • Step 1: proton transfer to the oxygen atom of the ether gives an oxonium ion : CH3 CH2 -O- CH2 CH3 + H + O H proton transfer H + CH3 CH2 -O- CH2 CH3 + : O H H H An oxonium ion • Step 2: nucleophilic displacement on the 1° carbon Br: - + + CH3 CH2 -O- CH2 CH3 H SN 2 CH3 CH2 -Br + : O-CH2 CH3 H • the alcohol is then converted to the alkyl bromide or iodide by another SN2 reaction 11-18 11 Cleavage of Ethers 3° and benzylic ethers are particularly sensitive to cleavage by HX • tert-butyl ethers are cleaved by HCl at room temp CH3 O-CCH3 + HCl CH3 SN 1 CH3 OH + Cl-C- CH 3 CH3 • in this case, protonation of the ether oxygen is followed by C-O cleavage to give the tert-butyl cation 11-19 11 Oxidation of Ethers Ethers react with O2 at a C-H bond adjacent to the ether oxygen to give hydroperoxides • reaction is by a radical chain mechanism OOH CH3 CH2 OCH 2 CH3 Diethyl ether Hydroperoxide: + O2 CH3 CH2 OCH CH 3 A hydroperoxide a compound containing the OOH group 11-20 11 Ethers - Protecting Grps When dealing with compounds containing two or more functional groups, it is often necessary to protect one of them (to prevent its reaction) while reacting at the other Suppose you wish to carry out this transformation HC CCH2 CH2 CH2 OH 4-Pentyn-1-ol ? CH3 CH2 C CCH2 CH2 CH2 OH 4-Heptyn-1-ol 11-21 11 Ethers - Protecting Grps • the new C-C bond can be formed by alkylation of the acetylide anion • the OH group, however, is more acidic (pKa 16-18) than the terminal alkyne (pKa 25) • treating the compound with one mole of NaNH2 will give the alkoxide anion rather than the acetylide HC CCH2 CH2 CH 2 OH + N a+ NH 2 4-Pentyn-1-ol HC CCH2 CH2 CH 2 O- Na + + N H3 11-22 11 Ethers - Protecting Grps A protecting group must be • easily added to the sensitive group • resistant to reagents used to transform the unprotected functional group • easily removed to regenerate the original functional group In this chapter, we discuss two -OH protecting groups • tert-butyl ether group • trimethylsilyl (TMS) group 11-23 11 Ethers - Protecting Grps The tert-butyl protecting group • formed by treatment of an alcohol with 2methylpropene in the presence of an acid catalyst HC CCH2 CH2 CH 2 OH 4-Pentyn-1-ol 1 . CH 2 = C( CH3 ) 2 H2 SO 4 CH3 HC CCH2 CH2 CH 2 OCCH3 CH3 11-24 11 Ethers - Protecting Grps • the unprotected alkyne is alkylated CH3 HC CCH2 CH2 CH 2 OCCH3 CH3 2 . Na + NH2 3 . CH 3 CH 2 Br CH3 CH3 CH2 C CCH2 CH2 CH 2 OCCH3 CH3 11-25 11 Ethers - Protecting Grps • the tert-butyl protecting group is removed by treatment with aqueous acid CH3 CH3 CH2 C CCH2 CH2 CH 2 OCCH3 4 . H3 O + / H2 O CH3 CH3 CH2 C CCH2 CH2 CH 2 OH + H2 C C( CH3 ) 2 4-Heptyn-1-ol 11-26 11 Ethers - Protecting Grps Trimethylsilyl (TMS) group • treat the alcohol with chlorotrimethylsilane in the presence of a 3° amine, such as triethylamine • the function of the 3° amine is to catalyze the reaction and to neutralize the HCl CH3 RCH2 OH + Cl-Si- CH3 ( CH3 CH2 ) 3 N CH3 Chlorotrimethylsilane CH3 RCH2 O- Si-CH3 CH3 A trimethylsilyl ether 11-27 11 Ethers - Protecting Grps • the TMS group is removed by treatment with aqueous acid or with F- in the form of tetrabutylammonium fluoride CH3 RCH2 O- Si-CH3 + H2 O CH3 H + CH3 RCH2 OH + HO- Si-CH3 CH3 A trimethylsilyl ether 11-28 11 Epoxides Epoxide: a cyclic ether in which oxygen is one atom of a three-membered ring • simple epoxides are named as derivatives of oxirane • where the epoxide is part of another ring system, it is shown by the prefix epoxy• common names are derived from the name of the alkene from which the epoxide is formally derived 2 H2 C 3 CH2 1O Oxirane (Ethylene oxide) H3 C H H C C CH3 O cis-2,3-Dimethyloxirane (cis-2-Butene oxide) 1 H O 2 H 1,2-Epoxycyclohexane (Cyclohexene oxide) 11-29 11 Synthesis of Epoxides-1 Ethylene oxide, one of the few epoxides manufactured on an industrial scale, is prepared by air oxidation of ethylene 2 CH2 = CH2 + O 2 Ag 2 H2 C CH2 O Oxirane (Ethylene oxide) 11-30 11 Synthesis of Epoxides-2 The most common laboratory method is oxidation of an alkene using a peroxycarboxylic acid (a peracid) O COOH O COOH CO Cl meta- chloroperoxybenzoic acid (MCPBA) O Mg 2 2+ O CH3 COOH Peroxyacetic acid (Peracetic acid) Magnesium monoperoxyphthalate (MMPP) 11-31 11 Synthesis of Epoxides-2 Epoxidation + Cyclohexene of cyclohexene O RCOOH CH2 Cl 2 A peroxycarboxylic acid H O H 1,2-Epoxycyclohexane (Cyclohexene oxide) + O RCOH A carboxylic acid 11-32 11 Synthesis of Epoxides-2 Epoxidation is stereospecific: • epoxidation of cis-2-butene gives only cis-2,3dimethyloxirane • epoxidation of trans-2-butene gives only trans-2,3dimethyloxirane H3 C CH3 C H C H cis-2-Butene RCO 3 H H3 C H C C CH3 H O cis-2,3-Dimethyloxirane 11-33 11 Synthesis of Epoxides-2 A mechanism for alkene epoxidation must take into account that the reaction • takes place in nonpolar solvents, which means that no ions are involved • is stereospecific with retention of the alkene configuration, which means that even though the pi bond is broken, at no time is there free rotation about the remaining sigma bond 11-34 11 Synthesis of Epoxides-2 A mechanism for alkene epoxidation R O R C O 3 2 H O C H O O 4 O 1 C C C C 11-35 11 Synthesis of Epoxides-3 A second general method involves 1. treatment of an alkene with Cl2 or Br2 in H2O to give a halohydrin; reaction is stereospecific 2. treatment of the halohydrin with base, causing an internal SN2 CH 3 CH= CH2 Propene Cl 2 , H2 O Cl CH 3 CH- CH 2 Na OH, H2 O HO 1-Chloro-2-propanol (a chlorohydrin) CH3 CH CH2 O Methyloxirane (Propylene oxide) 11-36 11 Synthesis of Epoxides-3 Problem: given the stereospecificity of this scheme, show that cis-2-butene gives cis-2,3-dimethyloxirane H H3 C H 1 . Cl 2 , H2 O C C CH3 2 . Na OH, H2 O cis-2-Butene H3 C H C C H CH3 O cis-2,3-Dimethyloxirane 11-37 11 Synthesis of Epoxides-4 Sharpless epoxidation • stereospecific and enantioselective R2 R1 T i( OiPr) 4 (+)-Diethyl tartrate R2 R1 + t e r t - BuOOH R3 CH2 OH O CH2 OH A R3 R2 (-)-Diethyl tartrate R1 O T i( OiPr) 4 R3 CH2 OH B 11-38 11 Reactions of Epoxides Because of the strain associated with the three-membered ring, epoxides readily undergo a variety of ring-opening reactions Nu C C O + HN u : C C HO 11-39 11 Reactions of Epoxides Acid-catalyzed ring opening • in the presence of an acid catalyst, epoxides are hydrolyzed to glycols O + H2 O Oxirane (Ethylene oxide) H+ HO OH 1,2-Ethanediol (Ethylene glycol) 11-40 11 Reactions of Epoxides Step 1: proton transfer to the epoxide gives a bridged oxonium ion intermediate H2 C CH2 : O + + H O H H CH2 + H2 O : H2 C O+ H Step 2: backside attack of H2O on the protonated epoxide opens the three-membered ring H H + H O : O H H2 C CH 2 O+ H H2 C CH 2 O: H 11-41 11 Reactions of Epoxides Step 3: proton transfer to solvent completes the hydrolysis H : O H H H +O H2 C CH2 H H2 C O: CH2 O O H H + + H O H H 11-42 11 Reactions of Epoxides Attack of the nucleophile on the protonated epoxide shows anti stereoselectivity • hydrolysis of an epoxycycloalkane gives a trans1,2-diol H O OH + H 1,2-Epoxycyclopentane (Cyclopentene oxide) H2 O H+ OH trans -1,2-Cyclopentanediol 11-43 11 Reactions of Epoxides Compare the stereochemistry of the glycols formed by these two methods H OH + RCO 3 H O H H2 O H OH trans-1,2-Cyclopentanediol OH OsO 4 , t-BuOOH OH cis-1,2-Cyclopentanediol 11-44 11 Reactions of Epoxides Ethers are not normally susceptible to attack by nucleophiles Because of the strain associated with the three-membered epoxide ring, epoxides undergo nucleophilic ring opening readily 11-45 11 Reactions of Epoxides Nucleophilic ring opening is stereospecific • attach of the nucleophile is anti to the leaving group H O + CH3 OH H Cyclohexene oxide - CH3 O N a + OH OCH3 trans-2-Methoxycyclohexanol 11-46 11 Reactions of Epoxides CH3 CH3 HOCH2 CH OH A glycol HSCH 2 CHOH A-mercaptoalcohol H2 O/ H3 O + N a+ SH - / H 2 O CH3 H2 C N a+ C N - / H 2 O CH3 N CCH2 CHOH A-hydroxynitrile CH3 CH O Methyloxirane HC CCH2 CHOH A-alkynylalcohol + 1 . HC C N a 2 . H2 O N H3 CH3 H2 N CH 2 CHOH A-aminoalcohol 11-47 11 Reactions of Epoxides Treatment of an epoxide with lithium aluminum hydride, LiAlH4, reduces the epoxide to an alcohol • the nucleophile attacking the epoxide ring is hydride ion, H:CH CH2 O Phenyloxirane (Styrene oxide) 1 . LiA lH4 2 . H2 O CH- CH 3 OH 1-Phenylethanol 11-48 11 Crown Ethers Crown ether: a cyclic polyether derived from ethylene glycol or a substituted ethylene glycol • the parent name is crown, preceded by a number describing the size of the ring and followed by the number of oxygen atoms in the ring O O O O O O 18-Crown-6 11-49 11 Crown Ethers The diameter of the cavity created by the repeating oxygen atoms is comparable to the diameter of alkali metal cations • 18-crown-6 provides very effective solvation for K+ 11-50 11 Thioethers The sulfur analog of an ether • IUPAC name: select the longest carbon chain as the parent and name the sulfur-containing substituent as an alkylsulfanyl group • common: list the groups bonded to sulfur followed by the word sulfide CH3 CH3 CH2 SCH2 CH3 CH3 CH2 SCHCH3 Ethylsulfanylethane (Dimethyl sulfide) 2-Ethylsulfanylpropane (Ethylisopropyl sjulfide) 11-51 11 Nomenclature Disulfide: contains an -S-S- group • IUPAC name: select the longest carbon chain as the parent and name the disulfide-containing substituent as an alkyldisulfanyl group • Common name: list the groups bonded to sulfur and add the word disulfide S H3 C S CH3 Methyldisulfanylmethane (Dimethyldisulfide) 11-52 11 Preparation of Sulfides Symmetrical sulfides: treat one mole of Na2S with two moles of an alkyl halide RSR + 2 N aX A sulfide 2 RX + N a2 S Cl Cl + 1,4-Dichlorobutane Na2 S SN 2 + 2 Na + Cl - S Thiolane (Tetrahydrothiophene) 11-53 11 Preparation of Sulfides Unsymmetrical sulfides: convert a thiol to its sodium salt and then treat this salt with an alkyl halide (a variation on the Williamson ether synthesis) + CH3I CH3(CH2)8CH2S- Na+ Sodium 1-decanethiolate SN2 CH3(CH2)8CH2SCH3 + Na+ I1-Methylsulfanyldecane (Decyl methyl sulfide) 11-54 11 Oxidation Sulfides Sulfides can be oxidized to sulfoxides and sulfones by the proper choice of experimental conditions S- CH3 Methyl phenyl sulfide H2 O2 25oC O N aIO 4 S- CH3 o 25 C Methyl phenyl sulfoxide O S- CH3 O Methyl phenyl sulfone 11-55 11 Prob 11.13 Account for the fact that tetrahydrofuran is considerably more soluble in water than diethyl ether. O Diethyl ether 8 g/100 mL water O Tetrahydrofuran very soluble in water 11-56 11 Prob 11.15 Which ethers can be prepared by a Williamson synthesis? For those can can’t, explain why not. CH3 (a) CH3 CH2 OCH CH 3 CH3 (b) CH3 COCH2 CH2 CH3 CH3 OCH3 (c) (e) CHCH3 OCH2 CH3 (d) (f) CH3 O CH2 OC( CH3 ) 3 11-57 11 Prob 11.16 Propose a mechanism for this reaction. CH= CH2 + CH3 OH H2 SO 4 OCH3 CHCH3 11-58 11 Prob 11.17 Draw structural formulas for the products when each compound is refluxed with concentrated HI. (a) O (b) O O (c) O (d) O 11-59 11 Prob 11.18 Write a pair of chain propagation steps for this radical chain reaction. Assume initiation is by a radical R•. Account for the regioselectivity of the hydroperoxidation. O + O2 Diisopropyl ether O O OH A hydroperoxide 11-60 11 Prob 11.20 Propose a mechanism for each reaction. H2 C CH2 + CH3 OH H2 SO 4 O Oxirane (Ethylene oxide) H2 C CH2 + CH3 CH2 OH O Oxirane (Ethylene oxide) CH3 OCH2 CH 2 OH 2-Methoxyethanol (Methyl Cellosolve) H2 SO 4 CH3 CH2 OCH 2 CH2 OH 2-Ethoxyethanol (Cellosolve) 11-61 11 Prob 11.21 Propose a mechanism for each step in this synthesis. O OH + HO HO H O + OH H + O O 1,4-Dioxane 11-62 11 Prob 11.22 Propose a synthesis of each compound from ethylene oxide and any readily available alcohols. (a) O O (b) O O O 11-63 11 Prob 11.25 How many stereoisomers are possible for 2-chloro1,2-diphenylethanol? Which of the possible stereoisomers are formed in this reaction? C6 H 5 C6 H5 H + HCl C H C O trans-2,3-Diphenyloxirane C6 H5 CH-CHC6 H 5 HO Cl 2-Chloro-1,2-diphenylethanol 11-64 11 Prob 11.26 Propose a mechanism for this rearrangement. O BF3 O Tetramethyloxirane 3,3-Dimethyl-2-butanone 11-65 11 Prob 11.27 Account for the stereospecificity of this epoxide ring opening reaction. CH3 + H2 O O H HO H2 SO 4 CH3 HO CH3 + HO H Only this glycol is formed HO H This glycol is not formed 11-66 11 Prob 11.28 If the starting alkene is trans, what is the configuration of the epoxide formed in each sequence? PhCH= CHPh 1,2-Diphenylethylene RCO 3 H O PhCH CHPh 2,3-Diphenyloxirane O 1 . Cl 2 , H2 O PhCH CHPh + 2 . CH 3 O N a 1,2-Diphenylethylene 2,3-Diphenyloxirane PhCH= CHPh 11-67 11 Prob 11.29 Show how this chiral epoxide can be prepared from an allylic alcohol precursor using the Sharpless epoxidation. O HO O O 11-68 11 Prob 11.30 Assume reaction here is by HOCl, which behaves as if it were Cl+OH-. Account for the observed regiochemistry and stereochemistry. CH3 CH3 R white blood cells HO Cholesterol CH3 CH3 OH R HO Cl 11-69 11 Prob 11.31 Propose a mechanism for this rearrangement. O O H2 SO 4 , TH F 11-70 11 Prob 11.34 Show how to convert cyclohexene to these compounds. (a) OH OH O (c) (b) OH (d) OH OH O N H2 (f) (e) OCH3 OH OCH3 Br (g) SCH2 CH 3 (h) (i) OH 11-71 11 Prob 11.34 (cont’d) Show how to convert cyclohexene to these compounds. (j) OH SH (k) (m) (l) CN Cl OH OH O O (n) HC(CH2 ) 5 CH C CCH3 11-72 11 Prob 11.35 Show reagents for each reaction. Br (c) (e) (b) Br (d) (a) O OH OH OH C CH (f) OH (g) OCH3 (i) (j) OH OH OH N (k) O H O H (h) O OCH3 11-73 11 Prob 11.36 Given this retrosynthetic analysis, propose a synthesis for the target molecule. OH O O + HO Cl Styrene 1-chloro-3-methyl2-butene 11-74 11 Prob 11.38 Propose structural formulas for A, B, C, and D, and the type of mechanism by which each is formed. CH 2 = CHCH 3 Propene Cl 2 heat Cl 2 , H2 O A ( C3 H5 Cl) C ( C3 H 7 ClO2 ) N aOH, H 2 O Ca( OH) 2 heat H2 O, HCl B ( C3 H6 O) D ( C3 H 6 O2 ) OH HOCH2 CHCH2 OH 1,2,3-Propanetriol (glycerol, glycerin) 11-75 11 Prob 11.39 Following are steps in the synthesis of gossyplure. OH 7 OH 8 + O 9 Br + HC CH 11-76 11 Prob 11.39 (cont’d) continuing from step 7 of the previous screen O C CH + Br 5 4 Br + HC CH Br OH 6-Bromo-1-hexanol 6 OH 7 11-77 11 Prob 11.39 (cont’d) continuing from step 4 of the previous screen OH 1 O 2 O 3 C CH + Br O 11-78 11 Prob 11.40 Propose a mechanism for each step and account for the regiospecificity of Steps 1 and 2. + Cl 2 500°C Step 1: Propene + Cl HCl 3-Chloropropene (Allyl chloride) OH Step 2:Cl + Cl 2 / H2 O Cl Cl + HCl OH Step 3: Cl Cl + Ca( OH) 2 O + CaCl 2 3-Chloro-1,2-epoxypropane (Epichlorohydrin) Cl 11-79 11 Ethers & Epoxides K+ End of Chapter 11 11-80