* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Xpert® C. difficile
Transmission (medicine) wikipedia , lookup
Globalization and disease wikipedia , lookup
Germ theory of disease wikipedia , lookup
Childhood immunizations in the United States wikipedia , lookup
Urinary tract infection wikipedia , lookup
Surround optical-fiber immunoassay wikipedia , lookup
Marburg virus disease wikipedia , lookup
Hepatitis C wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Carbapenem-resistant enterobacteriaceae wikipedia , lookup
Gastroenteritis wikipedia , lookup
Hepatitis B wikipedia , lookup
Schistosomiasis wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Neonatal infection wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
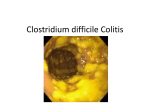
Xpert C.difficile ® Rapid and Accurate. It’s About Time. Xpert C. difficile ® Detection of Clostridium difficile in 45 minutes. In Vitro Diagnostic Medical Device A better way. “Patients infected with C. difficile are a source of transmission to other patients, therefore it is important that infected patients are detected as early as possible. Rapid molecular testing, providing wholly accurate results in 45 minutes, when combined with isolation of infected patients, provides an important new weapon in our battle against healthcare associated infections.” Professor Richard James Director of the Center for Healthcare Associated Infections University of Nottingham The Need The Solution C. difficile infections (CDI) have been increasing in incidence and severity, and are associated with an increase in length of hospital stays, costs, morbidity and mortality.1 CDI is also reported to be the most common cause of hospital acquired diarrhea and necessitates rapid and accurate diagnosis. Xpert® C. difficile detects the presence of toxin-producing Clostridium difficile in 45 minutes. Detection of three targets: Toxin B, Binary Toxin (cdtA), and tcdC deletion provides superior coverage and offers presumptive identification of the 027/NAP/BI epidemic strains. This new solution delivers both speed and accuracy and eliminates the need for additional or repeat testing. • In major EU countries CDI incidence is reported at 11 per 10,000 admissions2 • Highly virulent (027-NAP1-BI) strains have caused outbreaks of severe disease in Europe and North America — with mortality rates above 50%3 Incremental costs associated with C. difficile infection2 Incremental costs up to €15,000 per infection Contributing Cost Factors: • incremental LOS • isolation costs • environmental cleanup • additional therapies LACK OF RELIABLE AND TIMELY DIAGNOSIS, TREATMENT, AND INFECTION CONTROL CAN DRAMATICALLY INCREASE COSTS. Currently screening tests lack sensitivity and it can take up to 72 hours before a result becomes available to the physicians. This has forced clinicians to choose between speed or accuracy. • Clinicians can promptly administer therapy for improved patient outcomes • Timely implementation of infection control initiatives to reduce spread of infection within institution4 • Superior test sensitivity eliminates the need for additional or repeat testing CDI has become a substantive and growing burden in hospitalized patients prompting the need for earlier and accurate detection. The impact of Cepheid’s Xpert C. difficile test can be significant: with 45-minute detection, clinicians can now initiate therapy and appropriate infection prevention and control measures sooner, leading to better patient management. Performance Performance characteristics of Xpert® C. difficile were determined in a prospective investigation study by comparing Xpert C. difficile on the GeneXpert® System with broth enriched toxigenic culture. PERFORMANCE CHARACTERISTICS OF XPERT® C. DIFFICILE AS COMPARED TO TOXIGENIC CULTURE Toxigenic Culture Xpert C. difficile C. difficile pos negative Toxin B+ 316 117 negative 22 1841 sensitivity: 93.5% specificity: 94.0% Broad Coverage The only rapid test which identifies three targets: Toxin B, Binary Toxin, and tcdC deletion. XPERT C. DIFFICILE RESULT ALGORITHM Individual Targets and Combination Toxin B+ tcdC deletion Binary Toxin Internal Control negative – – –/+ + C. difficile positive + – – N/A C. difficile positive + – + N/A C. difficile positive + + – N/A Epidemic C. difficile positive + + + N/A tcdA tcdC Xpert C. difficile result PATHOGENICITY LOCUS tcdD tcdE tcdB regulator toxin b cdtR regulator repressor deletion 117 cdtA cdtB binary toxin targeted genes Unique Delivers immediate actionable results. PRESUMPTIVE POSITIVE TOXIGENIC C. DIFFICILE 027/NAP1/BI Rapid and Accurate. Xpert C. difficile ® • Fully automated process reduces handling time to just minutes • Random access for flexibility and workflow optimization • Rapid results to improve patient management • Fully integrated reagent and instrument system for accuracy and reproducibility WORKFLOW: 3 Easy Steps Total hands-on time: 1 Minute 1 2 3 Insert swab into Elution reagent vial and break at score Vortex and dispense Sample into Port S Insert cartridge and start assay. ORDERING INFORMATION Xpert® C. difficile (10 Cartridges with reagents) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Catalog No. GXCDIFFICILE-CE-10 References: 1. Zilberberg et al. Emerging Infectious Diseases, Vol 14, No 6, 2008 2. Kuijper et al. European Society of Clinical Microbiology & Infectious Diseases, CMI, 12 (Suppl 6), 2-18 3. Mundy et al. Infection 2007; 35: 300-307 4. National Prevalence Study of Clostridium Difficile in U.S. Healthcare Facilities. www. apic.org The molecular revolution is here. CORPORATE HEADQUARTERS EUROPEAN HEADQUARTERS 904 Caribbean Drive Sunnyvale, CA 94089 USA Vira Solelh 81470 Maurens-Scopont, France TOLL FREE +1.888.336.2743 +1.408.541.4191 FAX +1.408.541.4192 PHONE PHONE FAX EMAIL www.Cepheidinternational.com +33.563.82.53.00 +33.563.82.53.01 [email protected] A better way. THE PURCHASE OF THIS PRODUCT ALLOWS THE PURCHASER TO USE IT FOR THE PERFORMANCE OF DIAGNOSTIC SERVICES FOR HUMAN IN VITRO DIAGNOSTICS. NO GENERAL PATENT OR OTHER LICENSE OF ANY KIND OTHER THAN THIS SPECIFIC RIGHT OF USE FROM PURCHASE IS GRANTED HEREBY. NO OTHER RIGHTS ARE CONVEYED EXPRESSLY, BY IMPLICATION OR ESTOPPEL TO ANY OTHER PATENTS. FUTHERMORE, NO RIGHTS FOR RESALE ARE CONFERRED WITH THE PURCHASE OF THIS PRODUCT. 0014-05