* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Colegio Interamericano de Radiología
Heart failure wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
History of invasive and interventional cardiology wikipedia , lookup
Electrocardiography wikipedia , lookup
Coronary artery disease wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Myocardial infarction wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Cardiothoracic surgery wikipedia , lookup
Jatene procedure wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Cardiac surgery wikipedia , lookup
Pericardial heart valves wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
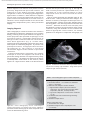
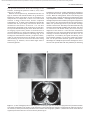
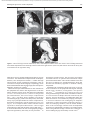
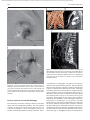
Document downloaded from http://www.elsevier.es, day 30/08/2011. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. Radiología. 2010;52(5):414-424 ISSN: 0033-8338 RADIOLOGÍA RADIOLOGÍA Publicación Oficial de la Sociedad Española de Radiología Médica Incluida en Index Medicus/MEDLINE www.elsevier.es/rx Actividad acreditada en base a la encomienda de gestión concedida por los Ministerios de Educación, Cultura y Deporte y de Sanidad y Consumo al Consejo General de Colegios Oficiales de Médicos con 1 crédito, equivalente a 4 horas lectivas. www.seram.es www.elsevier.es/rx UPDATE Radiological approach to cardiac tamponade J.R. Fortuño Andrésa,*, A. Alguersuari Cabiscola, J. Falcó Fagesa, E. Castañer Gonzálezb, P. Bermudez Bencerreya a Unitat de Radiologia Vascular i Intervencionista, Servei Diagnòstic per la Imatge, UDIAT-Centre Diagnòstic, Institut Universitari Parc Taulí-UAB, Sabadell, Barcelona, Spain b Sección de Radiología Torácica, Servei Diagnòstic per la Imatge, UDIAT-Centre Diagnòstic, Institut Universitari Parc Taulí-UAB, Sabadell, Barcelona, Spain Received 21 January 2010; accepted 21 May 2010 KEYWORDS Cardiac tamponade; Pericardium; Pericardiocentesis; Percutaneous drainage Abstract Cardiac tamponade is a life-threatening medical emergency. The radiologist’s role in the clinical management of patients with cardiac tamponade is usually minor and nearly always limited to the diagnostic process, and the condition is normally treated by other specialists. In this review, we aim to provide readers with the essential information to enable a complete diagnostic and therapeutic radiological approach. We emphasize US-guided percutaneous pericardial drainage; when performed correctly, this technique improves patients’ symptoms immediately with minimal discomfort and a very low rate of complications. © 2010 SERAM. Published by Elsevier España, S.L. All rights reserved. PALABRAS CLAVE Taponamiento cardiaco; Pericardio; Drenaje percutáneo Abordaje radiológico del taponamiento cardiaco Resumen El taponamiento cardiaco es una urgencia médica que puede comprometer la vida del paciente. Como radiólogos habitualmente tenemos una participación menor en el manejo clínico de esta enfermedad, casi exclusivamente limitada al proceso diagnóstico, mientras que el tratamiento lo realizan otros especialistas. Con esta revisión pretendemos dar las nociones básicas imprescindibles para realizar un abordaje radiológico completo tanto diagnóstico como terapéutico. Hacemos especial hincapié en la técnica de drenaje pericárdico percutáneo guiado por ecografía, que realizada correctamente proporciona al paciente una mejora sintomática instantánea con mínimas molestias y con muy baja tasa de complicaciones. © 2010 SERAM. Publicado por Elsevier España, S.L. Todos los derechos reservados. *Corresponding author. E-mail: [email protected] (J.R. Fortuño Andrés). 0033-8338/$ - see front matter © 2010 SERAM. Published by Elsevier España, S.L. All rights reserved. Document downloaded from http://www.elsevier.es, day 30/08/2011. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. Radiological approach to cardiac tamponade 415 Cardiac tamponade is a medical emergency that may be severe enough to threaten the life of the patient. 1 Understanding the pathophysiology of cardiac tamponade, which is a condition that has been known for centuries, 2 brings out the necessity for a decisive therapeutic intervention. As soon as the effects on the circulatory system secondary to an increase of pericardial pressure are diagnosed, urgent fluid drainage needs to be performed in order to restore heart function For effective diagnosis and treatment of cardiac tamponade, we need appropriate clinical orientation, understanding of the pathophysiology of the condition, imaging techniques such as chest radiography and ultrasound, and a percutaneous drainage system with echographic guidance. Surprisingly, although the diagnostic and therapeutic tools are available and are profusely used in other clinical scenarios, few radiologists are normally involved in the management of this condition. The aim of this update is to provide a comprehensible approach of the pathophysiological and diagnostic characteristics and treatment of cardiac tamponade, with an emphasis on the pericardial drainage technique, its outcomes and complications, and to consider other possible alternatives. In our opinion, radiologists should be aware not only of the radiologic signs of cardiac tamponade, but also should be prepared for treating this condition in an easy, rapid, safe and effective manner. Pressure Introduction Cardiac tamponade a b Limit of pericardial strech A B Volume Figure 1 Pericardial pressure-volume curve. As the pericardial effusion initially increases, the pericardial space accommodates the volume of fluid without a significant increase in pericardial pressure. When pericardial stretch limit and reserve volume are exceeded, even small increments of volume cause significant increase in the pericardial pressure. This fact leads to a decrease in diastolic heart filling which results in decreased stroke volume, and finally, reduced cardiac output. In rapid pericardial effusion (curve a) the capacity of the pericardium to stretch is lower, and therefore, the reserve volume is scarce (A). Small volumes may provoke tamponade, unlike slow or subacute effusion (curve b) where the pericardial capacity to stretch allows a more progressive accommodation to the expanding effusion, with a larger reserve volume (B). Pathophysiology Cardiac tamponade results from compression of the heart chambers secondary to accumulation of fluid, gas, blood or clots within the pericardial cavity. Understanding of the pathophysiological features of cardiac tamponade is essential to diagnosis and treatment. 3 The pericardium is a fibrous sac that contains the heart and that is composed of two layers: the internal or visceral, which is attached to the epicardium, and the external or parietal that consists of collagen and elastin fibers. Under normal conditions, the space between the two lawyers contains15–20 ml of serous fluid and is practically virtual in healthy people. Pericardial tissue determines the mechanical characteristics of the pericardium: at physiological cardiac volumes, the pericardium exhibits a compliant behaviour. The capacity of the pericardium to stretch increases progressively until it becomes stiff in a relatively abrupt manner. These characteristics determine the pressure-volume curve of the pericardium (fig. 1). If the volume increases slowly, the pericardium initially distends to a certain point without elevation of pericardial pressure, and since this pressure does not exceed cavity pressure, tamponade does not occur. 4 As the pericardial content progressively accumulates, the limit of the reserve volume is reached which is the volume that will just distend the pericardium without increasing pressure. Once this limit is exceeded, any increment provokes pericardial pressure increments. When this pressure exceeds intracardiac pressure, cardiac chambers collapse compromising heart filling. The rate of accumulation is the key to effective compensatory mechanisms of adaptation 5 and is more significant in establishing cardiac tamponade than a definite amount of pericardial fluid. This explains why patients with chronic effusion of gradual setting can tolerate accumulations of fluid of up to 1.000–1.500 ml. Hemodynamic compromise appears when the effusion reduces the cardiac chambers and complicates the ventricular filling, affecting cardiac output. Since right hearts works at lower pressures, it is more susceptible to increases of pericardial pressure; this is why the first hemodynamic consequence of tamponade is impairment of right chamber filling. Similarly, since the free ventricular walls cannot distend and the total cardiac work (blood volume) is constant, when the pericardial pressure increases, the interdependence between chambers also increases. Volume within a chamber increases reciprocally when pressure in the adjacent chamber decreases. This explains the effects of respiratory phases during tamponade. Under normal conditions, inspiration increases heart filling (mainly of right chambers) when intrathoracic pressure decreases, due to the gradient pressure between systemic veins and right cardiac chambers. During tamponade, Document downloaded from http://www.elsevier.es, day 30/08/2011. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. 416 however, since the increase of intrapericardial pressure limits the stretch capacity and filling of the right ventricle, when inspiration fills the right heart, this can only distend towards the left ventricle. This fact causes the so-called paradoxical septal motion that limits the filling of the left ventricle, which finally results in stroke volume and cardiac output involvement. These changes explain the clinical signs for the diagnosis of cardiac tamponade: 1. Jugular venous distention secondary to venous pressure increase due to impaired venous return to the right heart. 2. Pulsus paradoxus defined as a fall in systolic blood pressure of more than 10 mmHg during inspiration. This is mainly due to the paradoxical septal motion during inspiration when venous return increases and the rest of the cardiac walls cannot distend because of the effusion. The septal shifting towards the left ventricle reduces notably the stroke volume, resulting in decreased systolic pressure during inspiration. 4 3. Reflex tachycardia as a mechanism to compensate the decrease in stroke volume secondary to poor filling of the left ventricle. Finally, when these mechanisms can no longer maintain an adequate cardiac output, hypotension and shock occur. Although both cavities work at 15–20 mmHg pressures, in severe tamponade, normal filling pressures can result in tamponade, this is the so-called low-pressure tamponade. 6 This happens in hypovolemia, in the context of pre-existing pericardial effusion, when a small increase of pericardial pressure results in collapse of the heart chambers due to their low pressure and, therefore, clinical signs of tamponade appear with normal jugular venous pressure. 7 Causes Causes of cardiac effusion are summarized in table 1. The causes most likely to lead to tamponade are bacterial and mycobacterial infections, 8 HIV infection and neoplastic and hemorrhagic diseases. However, although idiopathic pericarditis is not likely to cause cardiac tamponade, given its high incidence, pericarditis is the most common cause. 9 J.R. Fortuño Andrés et al Table 1 Etiology of pericardial effusion Idiopathic Infectious 1. Viral: Echococcus, Coxsackie virus, Adenovirus, Cytomegalovirus, hepatitis B virus, infectious mononucleosis, HIV 2. Bacterial: Pneumococcus, Staphylococcus, Streptococcus, Mycoplasma, Lyme disease, Haemophilus influenzae, N. meningitidis 3. Micobacterium: M. tuberculosis, M. avium intracellulare 4. Fungal: histoplasmosis, Coccidioimycosis 5. Protozoal Immune-inflammatory 1. Connective tissue diseases: Lupus erythematosus, rheumatoid arthritis, scleroderma 2. Arteritis: Polyarterititis nodosa, temporal arteritis 3. Myocardial infarction • Early • Late (Dressler syndrome) 4. Post-traumatic/Postoperative 5. Drug-associated: Procainamide, hydralazine, isoniazid, ciclosporin, etc. Neoplastic 1. Primary: mesothelioma, sarcomas 2. Secondary: lung, breast, lynphomas, leukemias Trauma: penetrating wound, after cardiopulmonary resuscitation techniques Iatrogenic 1. Early post-surgical 2. Endovascular procedures: coronary angioplasty, defibrillators or pacemakers, placement of central catheters 3. Post radiation Congenital: cysts Other: 1. Heart failure 2. Hypothyroidism 3. Amyloidosis 4. Aortic dissection Clinical diagnosis Although cardiac tamponade is a clinical diagnosis, most signs and symptoms are not very specific when analyzed individually. 10 Reflex tachycardia secondary to low cardiac output is always present, as are the rest of the sympathetic mechanisms triggered to maintain a sufficient cardiac output. Hypotension is present in clinically significant tamponade with serious hemodynamic involvement; however, hypertensive patients might maintain high blood pressure. Pulsus paradoxus is a characteristic finding but it is nonspecific since other conditions such as massive pulmonary thromboembolism (PT) or severe bronchopulmonary disease 11 can also provoke it. High jugular venous pressure is also present, except in low-pressure tamponade. Electrocardiographic findings are relatively sensitive, although not sufficiently specific and result from acute pericarditis (diffuse ST segment elevation, PR segment depression) or from the pericardial effusion (low QRS voltages and electrical alternans). 12 Differential diagnosis must include constrictive and restrictive pericarditis, other causes of cardiogenic shock and right heart failure secondary to massive PT. Document downloaded from http://www.elsevier.es, day 30/08/2011. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. Radiological approach to cardiac tamponade Clinical diagnosis is not difficult if the patient has been previously diagnosed with pericardial effusion, has undergone a recent medical procedure suggesting iatrogenesis or there is an underlying pathology with a high incidence of effusion. 13 More difficult to diagnosis are the cases in which the background cannot help to predict effusion and tamponade, as in these cases it is essential a clinical suspicion based on the clinical data supported by complementary tests, mainly ultrasound imaging. Imaging diagnosis Chest radiography is considered normal if the effusion is not quantitatively important. Although not a very common fi nding, widening of the epicardial band can appear on lateral projections (the epicardial fat pad sign). In slow pericardial effusion, radiography demonstrates an enlarged cardiac silhouette with the characteristic water-bottle appearance (fig. 2). In these cases, comparing previous radiographs with the new ones can be most helpful in establishing diagnosis. In patients with pneumopericardium, radiographs show the heart s u r r o u n d e d b y a i r. T h e p r e s e n c e o f p e r i c a r d i a l calcifications suggests chronic effusion or pericardial constriction. M mode and 2D Doppler Ultrasound is the imaging technique of choice for diagnosis and follow-up of pericardial effusion because of its high sensitivity, safety and accessibility. 14 Ultrasound can determine the degree of hemodynamic involvement of the effusion, which appears as a hypo-anechoic band of variable thickness 417 between the two pericardial layers (fig. 3). Although the fluid is initially located at a posterobasal level, it is u n c ommon f or a n on -l oc u l ate d e f f u s ion to cause tamponade unless the fluid surrounds the heart completely. Signs of cardiac tamponade are abundant (table 2), but some of them —mainly transvalvular fl ow changes— are diffi cult to assess by radiologists without experience in echocardiography. Collapse of the right chambers during the filling phase —first affecting the atrium and then the ventricle—, distention of inferior vena cava (IVC), with 50 % diameter reduction during inspiration under normal conditions, along with moderate or severe effusion and within an appropriate clinical scenario, are reliable parameters for diagnosis of tamponade. Echography can Figure 3 Transthoracic ultrasound. Severe pericardial effusion in both sacs involving right chambers. Image shows partial collapse of right ventricle (asterisk). Table 2 Echocardiographic signs of cardiac tamponade 2D echographic signs 1. Pericardial effusion: > 2 cm effusion is considered severe. (↓ Sn and Sp) 2. Reduction and collapse of cardiac chambers • Right chambers collapse (atrial first and then ventricular): lack of these signs excludes cardiac tamponade. (NPV 99 %, Sn and Sp 50–60 %) • Left atrium compression: in more severe conditions. (↑↑ Sp) 3. ICV dilation with little variation during breathing. (↓ Sn and Sp) 4. Paradoxical septal motion F i g u r e 2 C h e s t r a d i o g r a p h y. S e v e r e c a r d i o m e g a l y (water-bottle heart) in a patient with chronic idiopathic pericardial effusion showing signs of cardiac tamponade in the context of disseminated lung cancer. Doppler echographic signs 1. Transvalvular flow changes 2. Paradoxical Doppler flow in suprahepatic veins (↓ Sn and SP) Sp: specificity; Sn: sensitivity; NPV: negative predictive value. Document downloaded from http://www.elsevier.es, day 30/08/2011. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. 418 also provide information about the etiology of the effusion, revealing the presence of fi brin, clots, tumor mass, air or calcium. Effusion located posteriorly, hematomas that are not hypo or anechoic and localized tumors can go unnoticed. Metastatic tumor infiltration of the pericardium can masquerade as tamponade if there is no associated effusion. In many of these cases, thoracic computed tomography (CT) 15 is useful in the evaluation of pericardial disease and also providing information about the mediastinal and thoracic structures. 16 CT can also differentiate tamponade from constrictive pericarditis, since pericardial thickening can mimic fluid collections. CT imaging is particularly useful in the detection of localized effusions or in areas inaccessible to ultrasound, since CT can be used as guidance for drainage (fig. 4). CT findings are distended IVC and SVC, periportal edema, refl ux of contrast material into the azygos vein and IVC and flattening of the left ventricular free wall during diastole (fig. 5). However, none of these signs alone is sufficiently specific. J.R. Fortuño Andrés et al Treatment Treatment of choice for cardiac tamponade is drainage of the pericardial content, preferably through percutaneous access. Medical management with fluid therapy and inotropic drugs is usually ineffective and should be used only as adjuvant treatment. 17 Although the first pericardial drainage was performed in 1810 by a surgeon in Napoleon’s army, and the first pericardiocentesis was performed in 1840, the optimal approach to pericardial drainage remains controversial. The ideal procedure should be easy and rapid, with minimal morbidity and mortality, low rates of recurrence and should ideally allow an etiologic diagnosis of the effusion. 18 The two best-established techniques are open surgical drainage and percutaneous drainage. Potential advantages of the latter are its promptness, accessibility and good tolerability, since percutaneous drainage is a very little painful procedure that can be performed without general anesthesia. Advantages of subxiphoid pericardial drainage are direct access to the pericardium with the possibility of obtaining Figure 4 a) Chest radiography. Patient with lung cancer in the upper right lobe (arrow) that progressed into pericardial effusion with cardiac tamponade in few months. b) The persistence of the symptoms after drainage is explained by the posterior location of the collection, which prevented the placement of the catheter with echographic guidance. c) Enhanced CT of the chest shows an organized pericardial collection. Access was done by fluoroscopy CT. Document downloaded from http://www.elsevier.es, day 30/08/2011. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. Radiological approach to cardiac tamponade 419 Figure 5 Patient with type A aortic dissection (a and c) with hemopericardium. Please note some of the CT findings associated with cardiac tamponade such as flattening of the free wall of the right ventricle (arrow in b) and contrast reflux into the IVC (a) due to involvement of the right heart filling. material in situ for etiologic diagnosis and the low rate of recurrence. Few studies have compared both techniques; however, two retrospective studies 18,19 confi rm the high effectiveness of both techniques with higher rates of recurrence in the percutaneous group. Surprisingly, one of these studies showed that surgical biopsy does not improve diagnostic accuracy of cytology. 18 Effectiveness of pericardiocentesis for the resolution of the symptoms was > 95 % in the largest series. In the two papers with the largest series that have been published ( 1 . 1 2 7 20 a n d 2 7 2 p a t i e n t s 21) a b o u t e c h o - g u i d e d pericardiocentesis, the rate of major complications was < 1,5 %, including heart chamber lacerations necessitating surgery, ventricular tachycardia, intercostal artery injury and pneumothorax. Minor complications, those that required only clinical monitoring, were < 5 % (fig. 6). If we compare these results with the results reported for blind pericardiocentesis —rates of total complications of 20 % and mortality rates as high as 6 % 22— it can be deduced that the use of echographic guidance improves signifi cantly the safety of the procedure. 23 In the Tsang’s extensive series, the recurrence rate within 6 months was 27 % for patients who underwent simple pericardiocentesis alone. This rate decreased to 14 % with the use of a catheter for extended drainage, which has become the mandatory approach in effusion causing tamponade. Although both techniques show high rates of success for the resolution of the symptoms and surgical drainage shows lower incidence, both guides 24 and opinion a r t i c l e s 19,20 r e c o m m e n d p e r i c a r d i o c e n t e s i s a n d echo-guided drainage as the initial treatment because it is a less invasive technique and well tolerated by the patient. Surgical drainage is recommended when the fluid collection is not accessible by imaging techniques, in recurrent effusion even undergoing an adequate treatment or when the patient undergoes surgery to solve an underlying condition, such as cardiac trauma. Even in the increasingly common cases of tamponade secondary to endoluminal procedures, pericardiocentesis with echo-guided drainage remains the treatment of choice making surgery unnecessary in most cases. 25 Document downloaded from http://www.elsevier.es, day 30/08/2011. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. 420 J.R. Fortuño Andrés et al Figure 7 a) 3D CT reconstruction of the chest depicting the subxiphoid and intercostal access used for pericardiocentesis and pericardial catheter placement. b) MIP CT of the chest in sagittal projection showing subxiphoid access. c) Sagittal MIP reconstruction of the chest in a patient with a pericardial drainage catheter. Figure 6 Caval-pericardial fistula as a complication of drainage catheter placement. Antero-posterior (a) and lateral (b) projections show opacification of IVC, right atrium and ventricle and pulmonary artery after injection of contrast agent via the catheter. The fistula self-resolved after removal of the catheter. How to perform pericardial drainage Percutaneous pericardial drainage is better performed under real-time echographic guidance. Use of CT-guided drainage is reserved for fluid collections that are not visible at echography or when echographic access is not possible. Fluoroscopic guidance is rarely used alone, but as a complement of echography. The patient lies supine in moderate anti-Trendelemburg position, which facilitates fluid to collect inferiorly in the pericardial sac and improves comfort in patients with shortness of breath. Vital signs monitoring (heart rate, blood pressure, oxygen saturation and continuous electrocardiographic monitoring) is mandatory and will provide us with full information about the hemodynamic status of the patient during the procedure. Even though the selection of the transducer depends on the availability, on the radiologist’s experience and patient’s morphotype, in our opinion, 4–5 MHz transducers are the most recommended since they have the perfect balance between depth, needed to visualize the pericardial space, and image resolution, essential for visualizing the needle trajectory. Subxiphoid approach is the most common, but intercostal approach is also used in some cases (fig. 7). Echography helps us to determine the Document downloaded from http://www.elsevier.es, day 30/08/2011. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. Radiological approach to cardiac tamponade best site for percutaneous puncture that allows reaching the pericardial area with the largest fluid accumulation without involving adjacent anatomical structures. Although percutaneous drainage can be performed by direct trocar insertion or the Seldinger micropunture technique, the latter is the safest and most recommended technique. Using one or the other depends on several factors, mainly the user experience, and in those cases with severe fluid accumulation and easy access, drainage can be performed using a 6 F catheter. In the case of small fluid collections or difficult access, a 20–22 G thin-wall needle is preferred. An exchange system with a 0.01899 guidewire, a coaxial introducer, a 0.03599 guide and a pigtail catheter are also used. Whatever the procedure and once the site of access is chosen, an echo-guided system is recommended in order to follow the needle and catheter trajectory to the fluid collection (fig. 8). After the usual sterilization procedures and local anesthesia administration, the percutaneous approach is performed. In most cases, following the needle until it reaches the sac is enough to be sure that we have reached the pericardium, which is verified by fluid flowing out. In neoplastic tamponade where the fluid is generally sanguineous, it can be difficult to determine whether it is pericardial effusion or blood coming from a potential cardiac perforation that could go unnoticed 26 (fig. 9). 421 Close observation of the fluid and absence of blood clotting characteristic of sero-hematic fluid will clear up any doubts. Electrocardiographic monitoring with an attachment to the needle can also help since direct myocardial stimulation with the needle and the visible Figure 9 Pericardial drainage and extraction of sanguineous fluid in a patient with advanced breast cancer. Figure 8 a) Equipment for direct trocar insertion (from right to left): 6 F pigtail catheter, scalpel, syringe with saline, local anesthesia. b) Echo-guided pericardial drainage performed using a coaxial technique with thin-wall needle. c) Final insertion. Document downloaded from http://www.elsevier.es, day 30/08/2011. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. 422 Figure 10 Pericardiography. Contrast agent diffusion within the pericardial sac. effects on cardiac rhythm can suggest an inadvertent cardiac puncture. Administration of a small amount of saline mixed with air, visible on US, can also help to confirm needle position. And last, if we combine an echographic and fluoroscopic approach, we can inject contrast agent and visualize its effusion within the pericardial space (fig. 10) under pericardiography or introduce a thin guidewire and see how it navigates. Once we have ascertained that the needle is intrapericardial, the fluid is aspirated and sent for biochemical, bacteriologic and cytological analyses. The thin guidewire, coaxial system and thick guidewire are then introduced and the catheter placed. There is no agreement on the ideal catheter size, but it seems to be agreed the use of 6 F and 10 F catheters for hemorrhagic or purulent tamponade, which might plug the catheter. Follow-up must be essentially clinic, assessing the improvement of the symptoms and the absence of fl ow from the catheter, using US examination only in patients with unsatisfactory outcome and when deciding to remove the catheter definitively. Sometimes the symptoms persist due to very dense or encapsulated effusions that are not accessible to drainage. In these cases the catheter may need to be repositioned under fl uoroscopy guidance, to be replaced for a thicker one or even introduction of a second catheter may be needed. The catheter will be removed based on the absence of symptoms and fluid at echography and absence of flow of fluids through the catheter itself. Neoplastic cardiac tamponade Cardiac tamponade associated with malignancies is characterized by a high recurrence rate that can reach up to 40 % if treated with percutaneous drainage alone. 27 The J.R. Fortuño Andrés et al most common neoplasias causing pericardial invasion are breast cancer, lung cancer, lymphoma, leukemia and melanoma, and secondary involvement is 40 times more frequent than primary involvement. Therapeutic aim for this kind of tamponade is the resolution of the symptoms and prevention of recurrences. Since tumoral invasion of the pericardium suggests bad prognosis —with an average survival of 6.1 months 28 and < 25 % one year after diagnosis 29 —, it is necessary to carefully assess the therapeutic management, and it seems logical to perform the less invasive and more comfortable procedure for the patiente. 30 Echo-guided pericardial drainage must be used to attain a prompt clinical improvement and, if short-term vital prognosis is not ominous, to instil sclerosing or cytotoxic agents such as thiotepa, 31 tetracicline, 32 doxycycline, bleomycin, 33 cisplatin, 34,35 carboplatin 36 or mitomycin C. Administration of these drugs via the catheter causes, besides local cytostatic effect, sclerosis of the pericardial blades as a result of the inflammatory reaction preventing recurrence of the effusion. Although not frequent, local pain, arrhythmia, fistula 37 and very rarely constrictive pericarditis can occur. However, it is an effective, safe and minimally invasive procedure. 38,39 Although there are not prospective studies that compare this technique with other more invasive procedures 40 such as subxiphoid pericardial w i n d o w, p l e u r o p e r i c a r d i a l w i n d o w o r b a l l o o n pericardiotomy, 41-44 it seems reasonable to perform an elective access using a less invasive technique with the lowest morbidity. In case of failure of pericardial drainage, and depending on the patient’s life expectancy, more complex procedures should be performed. 45-47 Conclusions Cardiac tamponade is a potentially life-threatening condition. The radiologist should be aware of the clinical, pathophysiological and radiologic signs of this entity in order to establish a diagnosis. Our role, however, should not be exclusively diagnostic and, in this sense, we should be able to treat tamponade effectively and safely performing echo-guided pericardial drainage. When performed under the best technical conditions, echo-guided drainage provides the patient with immediate symptomatic relief with minimal discomfort and a very low rate of complications. Conflict of interest The authors have no financial relationship to disclose. References 1. Spodick DH. Acute cardiac tamponade. N Engl J Med. 2003;349:684-90. 2. Meyer P, Keller PF, Spodick DH. Empress Sissi and cardiac tamponade: An historical perspective. Am J Cardiol. 2008; 102:1278-80. Document downloaded from http://www.elsevier.es, day 30/08/2011. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. Radiological approach to cardiac tamponade 3. Shabetai R, Hoit BD. Cardiac tamponade. UpTo-Date online (13.2). [accedssed 2007 Nov]. Available from: http://www. uptodate.com. 4. LeWinter MM, Kabbani S. Pericardial Diseases. In: Zipes PD, editors. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 7th ed. Elsevier Saunders; 2005. p. 1757-80. 5. Ameli S, Shah PK. Cardiac tamponade: pathophysiology, diagnosis, and management. Cardiol Clin. 1991;9:665-74. 6. Sagristà-Sauleda J, Angel J, Sambola A, Alguersuari J, Permanyer-Miralda G, Soler-Soler J. Low-pressure cardiac tamponade: clinical and hemodynamic profile. Circulation. 2006;114:945-52. 7. Lewinter MM. Pericardial diseases. In: Braunwald E, Zipes D, Libby P, editors. Heart Disease. 7th ed. Philadelphia: WB Saunders; 2007. p. 1763-9. 8. Reuter H, Burgess LJ, Louw VJ, Doubell AF. The management of tuberculous pericardial effusion: experience in 233 consecutive patients. Cardiovasc J S Afr. 2007;18:20-5. 9. Sagristà-Sauleda J, Angel J, Permanyer-Miralda G, Soler-Soler J. Long-Term Follow-Up of Idiopathic Chronic Pericardial Effusion. NEJM. 1999;341:2054-9. 10. Roy C, Minor M, Brookhart MA, Choudhry NK. Does this patient with a pericardial effusion have a cardiac tamponade. JAMA. 2007;297:1810-8. 11. Swami A, Spodick DH. Pulsus paradoxus in cardiac tamponade: A pathophysiologic continuum. Clin Cardiol. 2003;26:215-7. 12. Eisenberg MJ, de Romeral LM, Heidenreich PA, Schiller NB, Evans GT. The diagnosis of pericardial effusion and cardiac tamponade by 12-lead ECG. A technology assessment. Chest. 1996;110:318-24. 13. Fowler NO. Cardiac tamponade. A clinical or an echocardiographic diagnosis. Circulation. 1993;87:1738-41. 14. Mercé J, Sagistra-Sauleda J, Permanyer-Miralda G, Evangelista A, Soler-Soler J. Correlation between clinical and Doppler echocardiographic findings in patients with moderate and large pericardial effusion: Implications for the diagnosis of cardiac tamponade. Am Heart J. 1999;138: 759-64. 15. Restrepo CS, Lemos DF, Lemos JA, Velasquez E, Diethelm L, Ovella TA, et al. Imaging findings in cardiac tamponade with emphasis on CT. Radiographics. 2007;27:1595-610. 16. Chiles C, Woodard PK, Gutierrez FR, Link KM. Metastatic involvement of the heart and pericardium: CT and MR imaging. Radiographics. 2001;21:439-49. 17. Sagristà-Sauleda J, Angel J, Sambola A, Permanyer-Miralda G. Hemodynamic effects of volume expansion in patients with cardiac tamponade. Circulation. 2008;117:1545-9. 18. McDonald JM, Meyers BF, Guthrie TJ, Battafarano RJ, Cooper JD, Patterson GA. Comparison of open subxiphoid pericardial drainage with percutaneous catheter drainage for symptomatic pericardial effusion. Ann Thorac Surg. 2003;76: 811-5. 19. Allen KB, Faber P, Warren W, Shaar C. Pericardial effusion: Subxiphoid pericardiostomy versus percutaneous catheter drainage. Ann Thorac Surg. 1999;67:437-40. 20. Tsang TS, Enriquez-Sarano M, Freeman WK, Barnes ME, Sinak LJ, Gersh BJ, et al. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clin Proc. 2002;77:429-36. 21. Cho BC, Kang SM, Kim DH, Ko YG, Choi D, Ha JW, et al. Clinical and echocardiographic characteristics of pericardial effusion in patients who underwent echocardiographically guided pericardiocentesis: Yonsei Cardiovascular Center experience, 1993–2003. Yonsei Med J. 2004;45:462-8. 423 22. Suehiro S, Hattori K, Shibata T, Saskaki Y, Minamimura H, Kinoshita H. Echocardiography-guided pericardiocentesis with a needle attached to a probe. Ann Thorac Surg. 1996;61:741-2. 23. Fagan S, Chan KL. Pericardiocentesis. Blind no more!. Chest. 1999;116:275-6. 24. Sagristá J, Almenar L, Angel J, Bardají A, Bosch X, Guindo J. Guías de práctica clinica de la Sociedad Española de Cardiología en patología pericárdica. Rev Esp Cardiol. 2000;53:394-412. 25. Tsang TS, Freeman W, Barnes ME, Reeder GS, Packer DL, Seward JB. Rescue echocardiographically guided pericardiocentesis for cardiac perforation complicating catheter-based procedures. J Am Coll Cardiol. 1998;32: 1345-50. 26. Atar S, Chiu J, Forrester JS, Siegel RJ. Bloody pericardial effusion in patients with cardiac tamponade: is the cause cancerous, tuberculous, or iatrogenic in the 1990’s. Chest. 1999;116:1564-9. 27. Shahbaz CM, Fatimi S. Characteristics of recurrent pericardial effusions. Singapore Med J. 2007;48:725-8. 28. Yonemori K, Kunitoh H, Tsuta K, Tamura T, Arai Y, Shimada Y, et al. Prognostic factors for malignant pericardial effusion treated by pericardial drainage in solid-malignancy patients. Med Oncol. 2007;24:425-30. 29. Tsang TS, Seward JB, Barnes ME, Bailey KR, Sinak LJ, Urban LH, et al. Outcomes of primary and secondary treatment of pericardial effusion in patients with malignancy. Mayo Clin Proc. 2000;75:248-53. 30. Vaitkus PT, Herrmann HC, LeWinter MM. Treatment of malignant pericardial effusion. JAMA. 1994;272:59-64. 31. Martinoni A, Cipolla CM, Cardinale D, Civelli M, Lamantia G, Colleoni M, et al. Long-term results of intrapericardial chemotherapeutic treatment of malignant pericardial effusions with thiotepa. Chest. 2004;126:1412-6. 32. Shepherd FA, Ginsberg JS, Evans WK, Scott JG, Oleksiuk F. Tetracycline sclerosis in the management of malignant pericardial effusion. J Clin Oncol. 1985;3:1678-82. 33. Liu G, Crump M, Goss PE, Dancey J, Shepherd FA. Prospective comparison of the sclerosisng agents doxycycline and bleomycin for the primary management of malignat pericardial effusion and cardiac tamponade. J Clin Oncol. 1996;14:3141-7. 34. Bischiniotis TS, Lafaras CT, Platogiannis DN, Moldovan L, Barbetakis NG, Katseas GP. Intrapericardial cisplatin administration after pericardiocentesis in patients with lung adenocarcinoma and malignant cardiac tamponade. Hellenic J Cardiol. 2005;46:324-9. 35. Tomkowski WZ, Wis’niewska J, Szturmowicz M, Kuca P, Burakowski J, Kober J, et al. Evaluation of intrapericardial cisplatin administration in cases with recurrent malignant pericardial effusion and cardiac tamponade. Support Care Cancer. 2004;12:53-7. 36. Moriya T, Takiguchi Y, Tabeta H, Watanabe R, Kimura H, Nagao K, et al. Controlling malignant pericardial effusion by intrapericardial carboplatin administration in patients with primary non-small-cell lung cancer. Br J Cancer. 2000;83: 858-62. 37. Grbolar A, Qaradaghi L, Imren Y, Tasoglu I, Coskun E, Avci T. Bronchopericardial fistula, an unusual complication of oxytetracycline sclerosis therapy. Interact Cardiovasc Thorac Surg. 2008;7:280-1. 38. Girardi L, Ginsberg R, Burt M. Pericardiocentesis and intrapericardial sclerosis: Effective therapy for malignant pericardial effusions. Ann Thorac Surg. 1997;64:1422-8. 39. Reynen K, Kockeritz U, Strasser RH. Metastases to the heart. Ann Oncol. 2004;15:375-81. 40. Gross JL, Younes RN, Deheinzelin D, Diniz AL, Silva RA, Haddad FJ. Surgical management of symptomatic pericardial Document downloaded from http://www.elsevier.es, day 30/08/2011. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. 424 effusion in patients with solid malignancies. Ann Surg Oncol. 2006;13:1732-8. 41. Del Barrio LG, Morales JH, Delgado C, Benito A, Larrache J, Martinez-Cuesta A, et al. Percutaneous balloon pericardial window for patients with symptomatic pericardial effusion. Cardiovasc Intervent Radiol. 2002;25:360-4. 42. Wilkes JD, Fidias P, Vaickus L, Perez RP. Malignancy-related pericardial effusion. 127 cases from the Roswell Park Cancer Institute. Cancer. 1995;76:1377-87. 43. Navarro Del Amo LF, Córdoba Polo M, Orejas Orejas M, López Fernández T, Mohandes M, Iñíguez Romo A. Percutaneous balloon pericardiotomy in patients with recurrent pericardial effusion. Rev Esp Cardiol. 2002;55:25-8. J.R. Fortuño Andrés et al 44. Wang HJ, Hsu KL, Chiang FT, Tseng CD, Tseng YZ, Liau CS. Technical and prognostic outcomes of double-balloon pericardiotomy for large malignancy-related pericardial effusions. Chest. 2002;122:893-9. 45. Frankel K. Treating malignancy-related efusions. Chest. 2003;123:1775. 46. Maher EA, Shepherd FA, Todd TJ. Pericardial sclerosis as the primary management of malignant pericardial effusion and cardiac tamponade. J Thorac Cardiovasc Surg. 1996;112:637-43. 47. Anderson TM, Ray CW, Nwogu CE, Bottiggi AJ, Lenox JM, Driscoll DL, et al. Pericardial catheter sclerosis versus surgical procedures for pericardia effusions in cancer patients. J Cardiovasc Surg (Torino). 2001;42:415-9.