* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download PDF - ClaimSecure
Survey
Document related concepts
Pharmacognosy wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Drug design wikipedia , lookup
Drug interaction wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Neuropharmacology wikipedia , lookup
Drug discovery wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Adherence (medicine) wikipedia , lookup
Transcript
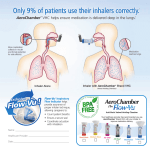
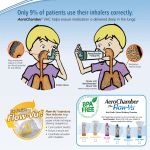
VOLUME X, ISSUE 2 Recently Introduced Products Drug Name Diamicron MR 60mg Indication Control of hyperglycemia Potential Impact Expected Avg. Annual Cost $ $47.45-$186.15 $: Est. drug plan expenditure increase of <1%* $$: Est. drug plan expenditure increase of 1-5%* $$$: Est. drug plan expenditure increase of >5%* Zenhale® – A new treatment alternative for asthma ® Zenhale (mometasone furoate/formoterol dihydrate) is an inhaler for the maintenance treatment of patients with asthma who cannot be effectively managed on asthma controller medications. Asthma is an inflammatory disorder of 1 the airways which leads to symptoms such as wheezing, coughing, shortness of breath and chest tightness. The Asthma Society of Canada reports that asthma is the leading cause of absenteeism from school and the third leading cause of work loss. In Ontario alone, more than one million people are affected by this chronic condition. Asthma is an incurable condition, thus medications control the disease by reducing the frequency, severity and length of symptomatic attacks. The Canadian guidelines advocate a stepwise approach for the management of asthma based on the severity of the disease. Mild asthma is treated with short acting beta agonists, e.g. Ventolin with the addition of inhaled corticosteroids, e.g. Flovent if symptoms persist. If further control is required, a combination inhaler that 2 contains both an inhaled corticosteroid and a long acting beta agonist, e.g. Advair, Symbicort is introduced. ® Zenhale should not be administered to patients who are adequately controlled or have not optimally utilized initial ® maintenance treatments. Zenhale is not a rescue medication; for relief of acute asthma symptoms, a short acting medication such as Ventolin must be used. This combination inhaler expands the airway and decreases inflammation, and similar to Advair and Symbicort, it functions as a second-step maintenance treatment. One study demonstrated ® that Zenhale provided comparable effectiveness with Advair in terms of lung function and clinical outcomes while 3 providing significantly faster action on airway dilation. ® Each Zenhale inhaler provides 120 doses and is available in three strengths, low dose (50mcg/5mcg), medium dose ® (100mcg/5mcg) and high dose (200mcg/5mcg). Zenhale is administered as two inhalations twice daily and delivers 30 days of therapy at a cost of $66.90, $84.90 and $102.90 for the low, medium, and high dose respectively. Since ® Zenhale is competitively priced and provides similar benefits as other combination inhalers; this medication will be fully covered by both Open and Managed Drug Formularies, and the Specialty Drug Programs. ® If you require additional information about Zenhale please contact Lavina Viegas, Clinical Pharmacist, Clinical Services Department, at (905) 949-3031 or 1-888-479-7587 ext. 3031. Recommendation: Full Coverage ClaimSecure reserves the right to amend in part or in its entirety stated special authorization clinical guidelines 1. 2. 3. References: Asthma Society of Canada. http://www.asthma.ca/adults/about/ March 3, 2011. 2010 Canadian Thoracic Society Guideline. http://www.respiratoryguidelines.ca/sites/all/files/cts_asthma_consensus_su mmary_2010.pdf March 3, 2011 ® Zenhale Private Payer Submission Binder. Merck, February 2011. * Based on the Financial Impact Analysis per 100 000 lives covered © 2011 ClaimSecure Inc.