* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 1% - ISpatula
Survey
Document related concepts
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Prescription costs wikipedia , lookup
Metalloprotease inhibitor wikipedia , lookup
Discovery and development of angiotensin receptor blockers wikipedia , lookup
Atypical antipsychotic wikipedia , lookup
5-HT2C receptor agonist wikipedia , lookup
Drug interaction wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Norepinephrine wikipedia , lookup
Cannabinoid receptor antagonist wikipedia , lookup
Chlorpromazine wikipedia , lookup
Nicotinic agonist wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Transcript
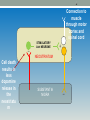
CHAPTER 28 Pharmacologic Management of Parkinson disease (PD) 2 Overview of Parkinson's Disease • PD is a progressive neurological disorder of muscle movement, characterized by combination of rigidity, bradykinesia, resting tremor, and postural instability • It generally affects the elderly and is estimated to afflict more than 1% of individuals over the age of 65 • PD is correlated with destruction of dopaminergic neurons in the substantia nigra with a consequent reduction of dopamine actions in the corpus striatum-parts of the brain's basal ganglia system that are involved in motor control 3 Overview of Parkinson's Disease • Thus, the normal modulating inhibitory influence of dopamine on cholinergic neurons in the neostriatum is significantly diminished, resulting in overproduction or a relative over-activity of acetylcholine by the stimulatory neurons • This triggers a chain of abnormal signaling, resulting in loss of the control of muscle movements 4 Connection to muscle through motor cortex and spinal cord _ STIMULATORY Ach NEURONE + NEOSTRIATUM Cell death results in less dopamine release in the neostriatu m SUBSTANTIA NIGRA _ 5 Strategy of treatment • Since there is no cure for PD, the aim of pharmacological therapy is to provide symptomatic relief • This is obtained re-establishing the correct dopamine/acetylcholine balance through the use of drugs that either increase dopaminergic actions or antagonizing the excitatory effect of cholinergic neurons 6 Drugs Used in Parkinson's Disease • Drugs used in Parkinson’s disease include: 1) Levodopa 2) Dopamine agonists 3) Monoamine oxidase (MAO) inhibitors 4) Cathechol-O-methyl transferase (COMT( inhibitors 5) Anticholinergic agents 6) Amantadine 7 Levodopa • Levodopa is the single most effective agent in the • • • • • treatment of PD It is immediate metabolic precursor of DA Levodopa is itself largely inert levodopa, as an a.a, is transported into the brain by a.a transport systems Therapeutic and adverse effects of levodopa result from its decarboxylation to dopamine Oral levodopa is absorbed rapidly from the small intestine by the transport system for aromatic amino acids 8 Aromatic Amino Acid Decarboxylase Dopamine DOPA Tyrosine Hydroxylase Tyrosine H+ DA DOPAC Uptake 1 HVA DA HVA Uptake 2 Dopamine receptor HVA DA 3MT 9 Levodopa • Certain a.as from ingested food can compete with levodopa for absorption from gut & for transport from blood to brain • About 1–3% of administered levodopa actually enters the brain unaltered; the remainder is metabolized extracerebrally, predominantly by decarboxylation to dopamine, which does not penetrate the BBB • It is combined with a peripheral dopa decarboxylase inhibitor (e.g. carbidopa) 10 Carbidopa • A dopa decarboxylase inhibitor that does not cross the BBB • Carbidopa diminishes the metabolism levodopa in the GIT and peripheral tissues: of a. Increases the availability of levodopa to the CNS b. Lowers the dose of levodopa needed by about 10- fold c. Decreases the severity of the side effects arising from peripherally formed dopamine 11 Levodopa (Clinical use) • Levodopa is widely used for treatment of all types of PD except those associated with antipsychotic drug therapy • Levodopa substantially reduces the severity of all the signs and symptoms of PD in the first few years of treatment • Patients then typically experience a decline in response during the third to fifth year of therapy (progression of the disease and the loss of striatal dopamine nerve terminals) 12 Levodopa ADRs A. Gastrointestinal tract (GIT) • Anorexia, nausea, and vomiting (likely due to dopamine’s stimulation of the CTZ) • Minimized by taking the drug in divided doses, with or immediately after meals, and by increasing the total daily dose very slowly • When levodopa is given in combination with carbidopa, adverse GIT effects are much less frequent & patients can tolerate proportionately higher doses 13 Levodopa ADRs B. Cardiovascular effects • Cardiac arrhythmias: caused by increased catecholamine formation peripherally • Postural hypotension as a result of the peripheral decarboxylation of levodopa and release of dopamine into the circulation • Incidence can be reduced if levodopa is taken in combination with Carbidopa 14 Levodopa ADRs C. Dyskinesias • Occur in up to 80% of patients receiving levodopa within 2 years of starting levodopa therapy • Are excessive and abnormal choreiform/ involuntary movements of the limbs, hands, trunk, and tongue • The development of dyskinesias is dose-related that occur most often concentration is high when the plasma levodopa • There is considerable individual variation in the dose required to produce them 15 Levodopa ADRs E. Fluctuations in Response • Certain fluctuations in clinical response to levodopa occur with increasing frequency as treatment continues I. Wearing-off phenomenon/ end-of-dose akinesia: • Related to the timing of levodopa intake • Each dose of levodopa effectively improves mobility for a period of time (1–2 hrs), but rigidity and akinesia return rapidly at the end of the dosing interval • Increasing the dose and frequency of administration can improve this situation 16 Levodopa ADRs E. Fluctuations in Response II. On-off phenomenon • Fluctuations in response are unrelated to timing of doses • Patients fluctuate rapidly between being “off,” having no beneficial effects from their medications, and being “on” but with disabling dyskinesias • Can be reduced by: *using a sustained-release formulation, **division of the total daily dose into more frequently administered portions, ***coadministration of COMT inhibitors or ****selegline, and *****regulation of dietary protein intake 17 Levodopa ADRs F. Behavioral Effects • Depression, anxiety, agitation, insomnia, somnolence, confusion, delusions, hallucinations, nightmares, euphoria, and other changes in mood or personality • More common in patients taking levodopa in combination with carbidopa • The use the “atypical” antipsychotic agents (e.g., clozapine), which are effective in the treatment of psychosis but do not cause or worsen parkinsonism 18 Drug Interactions of levodopa • Pyridoxine (vitamin B6): increase extracerebral metabolism of levodopa and may prevent its therapeutic effect unless carbidopais is also taken • Nonspecific MAO inhibitors (e.g. phenelzine): • Accentuates the actions of levodopa and may precipitate life-threatening hypertensive crisis • MAO inhibitors must be discontinued at least 14 days before levodopa is administered 19 Levodopa • Contraindications Psychotic patients 2) Angle-closure glaucoma1 1) • Careful management in patients with: a) History of cardiac arrhythmias or recent cardiac infarction b) Active peptic ulcer must be managed carefully c) History of melanoma or with suspicious undiagnosed skin lesions2 20 Monoamine Oxidase Inhibitors • Two types of monoamine oxidase have been distinguished in the nervous system (MAO-A and MAO-B) • The isoenzyme MAO-B is the predominant form in the striatum and is responsible for most of the oxidative metabolism of dopamine in the brain • Blockade of dopamine metabolism makes more dopamine available for stimulation of its receptors • Selective MAO-B Inhibitors: Selegiline & Rasagiline 21 Monoamine Oxidase Inhibitors • Neither selegiline nor rasagiline should be taken by patients receiving meperidine agitation, and hyperthermia) (stupor, rigidity, • They should be used with care in patients receiving TCAs or SSRIs b/c of the theoretical risk of acute toxic interactions of the serotonin syndrome type * • ADEs: o Most related to the increased levels of dopamine o Anxiety, insomnia (selegline) 22 Catechol-O-Methyltransferase Inhibitors • Normally, the methylation of levodopa by catechol-O- methyltransferase (COMT) to 3-O-methyldopa is a minor pathway for levodopa metabolism • However, when peripheral dopamine decarboxylase activity is inhibited by carbidopa, a significant concentration of 3-O-methyldopa is formed that competes with levodopa for active transport into the CNS • Selective COMT inhibitors: tolcapone & entacapone 23 Effect of entacapone on dopa concentration in the central nervous system (CNS). COMT = catechol-Omethyltransferase 24 Catechol-O-Methyltransferase Inhibitors • Entacapone is generally preferred: tolcapone has both central & peripheral effects, whereas effect of entacapone is peripheral • ADRs: o Increased levodopa exposure o Orange discoloration of urine o Hepatoxicity (tolcapone) 25 Dopamine Receptor Agonists • Dopamine agonists may delay the need to employ levodopa therapy in early PD and may decrease the dose of levodopa in advanced Parkinson's disease • Dopamine receptor agonists: Ergot derivatives e.g. bromocriptine 2. Non-ergot derivatives e.g. apomorphine, pramipexole, ropinirole, and rotigotine 1. • The differences between the ergot derivatives and the newer/ non-ergot agents reside primarily in their adverse effects, tolerability, and speed of titration 26 Dopamine Receptor Agonists ADRs A. Gastrointestinal tract effects: • Anorexia, N & V: can be minimized by taking the medication with meals • Constipation, dyspepsia, and symptoms of reflux • Bleeding from peptic ulceration 27 Dopamine Receptor Agonists ADRs B. Cardiovascular effects: • Postural hypotension common at the initiation of therapy especially with the ergot derivatives • Dose-related painless digital vasospasm with the ergot derivatives • Cardiac arrhythmias (discontinuation) • Peripheral edema 28 Dopamine Receptor Agonists ADRs C. Dyskinesias: reversed by reducing the total dose of dopaminergic drugs D. Mental Disturbances • Include confusion, hallucinations, delusions, and other psychiatric reactions which are more common and severe with dopamine receptor agonists than with levodopa • Disappear on withdrawal 29 Dopamine Receptor Agonists ADRs E. Miscellaneous Adverse Effects (ergot derivatives) o Headache o Nasal congestion o Increased arousal o Pulmonary infiltrates o Pleural & retroperitoneal fibrosis o Erythromelalgia (red, tender, painful, swollen feet &, occasionally, hands, at times associated with arthralgia) 30 31 Dopamine Receptor Agonists Contraindications 1. History of psychotic illness 2. Recent myocardial infarction 3. Active peptic ulceration 4. Peripheral vascular disease (ergot derivatives) 32 Acetylcholine-Blocking Drugs • Centrally acting antimuscarinic drugs include: benzotropine mesylate, biperiden, orphenadrine, procyclidine, & trihexphenidyl • Their efficacy in PD is likely due to the ability to block muscarinic receptors in the striatum • Are much less efficacious than levodopa and thus are most commonly used during the early stages of the disease or as an adjunct to levodopa therapy 33 Acetylcholine-Blocking Drugs ADRs • The adverse effects of these drugs are a result of their anticholinergic properties (CNS & peripheral effects) 1. CNS: mental confusion, delirium, and hallucinations 2. Peripheral: constipation, urinary retention, and blurred vision through cycloplegia, sinus tachycardia, & dry mouth • Are contraindicated in patients with glaucoma, prostatic hyperplasia, or pyloric stenosis Amantadine • An antiviral agent used for the prophylaxis and treatment of influenza A • Its MOA in parkinsonism is unclear. However it may: a. Potentiate dopaminergic function by influencing the synthesis, release, or reuptake of dopamine b. Has anticholinergic properties c. Block NMDA glutamate receptors d. Antagonize the effects of adenosine at adenosine A2A receptors, which are receptors that may inhibit D2 receptor function • Benefits are short-lived, often disappearing after only few weeks Amantadine ADRs 1) CNS: headache, restlessness, depression, irritability, 2) 3) 4) 5) 6) 7) 8) insomnia, agitation, excitement, hallucinations, & confusion Acute toxic psychosis & convulsions (overdosage) Livedo reticularis Peripheral edema HF Postural hypotension Anticholinergic: urinary retention, constipation, and dry mouth GI disturbances: anorexia & nausea 36 Livedo reticularis