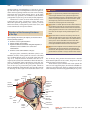
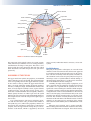
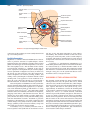
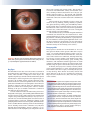
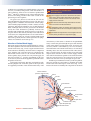
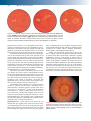
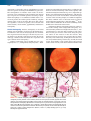
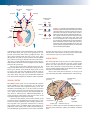
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Special Sensory Function Special Sensory Function
Survey
Document related concepts
Fundus photography wikipedia , lookup
Contact lens wikipedia , lookup
Blast-related ocular trauma wikipedia , lookup
Retinal waves wikipedia , lookup
Keratoconus wikipedia , lookup
Idiopathic intracranial hypertension wikipedia , lookup
Vision therapy wikipedia , lookup
Visual impairment wikipedia , lookup
Dry eye syndrome wikipedia , lookup
Photoreceptor cell wikipedia , lookup
Eyeglass prescription wikipedia , lookup
Cataract surgery wikipedia , lookup
Corneal transplantation wikipedia , lookup
Macular degeneration wikipedia , lookup
Retinitis pigmentosa wikipedia , lookup
Mitochondrial optic neuropathies wikipedia , lookup
Transcript
U N I T Special Sensory Function O f all the ancient civilizations, it was the Greeks who led the way in understanding the body and its workings. One of the earliest Greek anatomists was Alcmaeon of Croton (c. 500 BC). Through his animal dissections, Alcmaeon came to recognize many structures and was the first to mention the eye in his writings. He described the optic nerve and decided that three things were necessary for vision— external light, the “fire” in the eye (he assumed there must be fire in the eye because a blow to the eye produces sparks, or stars), and the liquid in the eyeball. The Greeks also developed early surgical procedures, among them techniques for the removal of cataracts. However, it was the Roman encyclopedist, Aulus Cornelius Celsus (1st century AD), whose most important surviving works are concerned with medicine, who provided a vivid description of the procedure: The needle is to be sharp enough to penetrate, yet not too fine; and this to be inserted straight through . . . at a spot between the pupil of the eye and the angle adjacent to the temple, away from the middle of the cataract, in such a way that no vein is wounded. The needle, however, should not be inserted timidly. When the spot is reached, the needle is to be sloped against the colored area [lens] itself and rotated gently, guiding it little by little below the pupil; when the cataract has passed below the pupil, it is pressed upon more firmly in order that it may settle below. XIII CHAPTER 54 Disorders of Visual Function Edward W. Carroll Carol M. Porth Robin L. Curtis DISORDERS OF THE ACCESSORY STRUCTURES OF THE EYE Disorders of the Eyelids Eyelid Weakness Eyelid Inflammation Disorders of the Lacrimal System Dry Eyes Dacryocystitis DISORDERS OF THE CONJUNCTIVA, CORNEA, AND UVEAL TRACT Disorders of the Conjunctiva Allergic Conjunctivitis Infectious Conjunctivitis Ophthalmia Neonatorum Disorders of the Cornea Corneal Trauma Keratitis Abnormal Corneal Deposits Corneal Transplantation Disorders of the Uveal Tract Uveitis The Pupil and Pupillary Reflexes INTRAOCULAR PRESSURE AND GLAUCOMA Control of Intraocular Pressure Glaucoma Angle-Closure Glaucoma Open-Angle Glaucoma Congenital and Infantile Glaucoma DISORDERS OF THE LENS AND LENS FUNCTION Disorders of Refraction and Accommodation Disorders of Refraction Disorders of Accommodation Cataracts Causes and Types of Cataracts Manifestations Diagnosis and Treatment DISORDERS OF THE VITREOUS AND RETINA Disorders of the Vitreous Disorders of the Retina The Neural Retina Photoreceptors Disorders of Retinal Blood Supply Retinopathies Retinal Detachment Macular Degeneration DISORDERS OF NEURAL PATHWAYS AND CORTICAL CENTERS Optic Pathways Visual Cortex Visual Fields Visual Field Defects Disorders of the Optic Pathways Disorders of the Visual Cortex Testing of Visual Fields DISORDERS OF EYE MOVEMENT Extraocular Eye Muscles and Their Innervation Eye Movements and Gaze Strabismus Paralytic Strabismus Nonparalytic Strabismus Diagnosis and Treatment Amblyopia A bout 70% of all sensory receptors are in the eyes. The photoreceptors sense and encode the patterns made by light in our surroundings. Neural pathways carry the coded information from the eyes to the brain where the signals gain meaning, fashioning images of the world around us. Almost 17.3 million persons in the United States have some degree of visual impairment; of these, 1.1 million are legally blind.1 The prevalence of vision impairment 1291 1292 UNIT XIII Special Sensory Function increases with age. An estimated 26% of persons 75 years of age and older report visual impairment severe enough to interfere with recognizing a friend across the room or reading newspaper print even when wearing glasses. At the other end of the age spectrum, an estimated 95,100 children younger than 18 years of age are severely visually impaired.1 Alterations in vision can result from disorders of the eyelids and optic globe (conjunctiva, cornea, and uvea), intraocular pressure (glaucoma), lens (cataract), vitreous humor and retina, visual pathways and visual cortex, and extraocular muscles and eye movement. Disorders of the Accessory Structures of the Eye VISION ➤ Vision is a special sensory function that incorporates the visual receptor functions of the eyeball, the optic nerve, and visual pathways that carry and distribute sensory information from the optic globe to the central nervous system, and the primary and visual association cortices that translate the sensory signals into visual images. ➤ The eyeball is a hollow spherical structure that functions in the reception of the light rays that provide the stimuli for vision. The refractive surface of the cornea and accommodative properties of the lens serve to focus the light signals from near and far objects on the photoreceptors in the retina. ➤ Visual information is carried to the brain by axons of the retinal cells that form the optic nerve. The two optic nerves fuse in the optic chiasm, where axons of the nasal retina of each eye cross to the contralateral side and travel with axons of the ipsilateral temporal retina to form the fibers of the optic radiations that travel to the visual cortex. After completing this section of the chapter, you should be able to meet the following objectives: ✦ State the cause of eyelid weakness ✦ Define entropion and ectropion ✦ Explain the differences between marginal blepharitis, a ➤ Binocular vision depends on the coordination of three pairs of extraocular nerves that provide for the conjugate eye movements, with optical axes of the two eyes maintained parallel with one another as the eyes rotate in their sockets. hordeolum, and a chalazion as to causes and manifestations ✦ State the causes and treatment of dry eye The optic globe, commonly called the eyeball, is a remarkable, mobile, nearly spherical structure contained in a pyramid-shaped cavity of the skull called the orbit (Fig. 54-1). Only the anterior one fifth of the orbit is occupied by the eyeball; the remainder is filled with muscles, nerves, the lacrimal gland, and adipose tissue that supports the normal position of the optic globe. Exposed surfaces of the eyes are protected by the eyelids, which are mucous membrane–lined skin flaps that provide a means for shutting out most light. Tears bathe the anterior sur- face of the eye; they prevent friction between it and the lid, maintain hydration of the cornea, and protect the eye from irritation by foreign objects. Three distinct layers form the wall of the eyeball: the sclera or outer supporting layer, the choroid or middle vascular layer, and the retina, which is composed of the neuronal retinal layer and the outer pigmented layer (Fig. 54-2). Retina Choroid Superior rectus Sclera Bulbar and palpebral conjunctiva Cornea Levator palpebrae superioris Lens Superior tarsal plate Iris Optic nerve Meibomian gland in tarsal plate Inferior rectus Inferior oblique muscle Orbicularis oculi muscle Ciliary body FIGURE 54-1 Lateral view of the eye and its appendages. CHAPTER 54 Disorders of Visual Function 1293 Pupil Anterior chamber Iris Cornea Conjunctiva Canal of Schlemm Posterior chamber Ciliary body Lens Cillary processes Vitreous body Retina Choroid Retinal blood vessel Optic disk Sclera Lateral rectus muscle Medial rectus muscle Optic nerve Fovea centralis FIGURE 54-2 Transverse section of the eyeball. The outer layer of the eyeball consists of a tough, opaque, white, fibrous layer called the sclera. It is strong yet elastic, and maintains the shape of the globe. The sclera is continuous with the cornea anteriorly and with the cranial dural sheath that surrounds and protects the optic nerve posteriorly. DISORDERS OF THE EYELIDS The upper and lower eyelids, the palpebrae, are modified folds of skin that protect the eyeball. The palpebral fissure is the oval opening between the upper and lower eyelids. At the corners of the eye, where the upper and lower lids meet, is an angle called the canthus; the lateral canthus is the outer, or temporal, angle, and the medial canthus is the inner, or nasal, angle. In each lid, a tarsus, or plate of dense connective tissue, gives the lid its shape (see Fig. 54-1). Each tarsus contains modified sebaceous glands, called meibomian glands, the ducts of which open onto the eyelid margins. Sebaceous secretions of the meibomian glands enable airtight closure of the lids and prevent rapid evaporation of tears. Two striated muscles, the levator palpebrae superioris and the orbicularis oculi, provide for movement of the eyelids (Fig. 54-3). The levator palpebrae, which is innervated by the oculomotor cranial nerve (cranial nerve [CN] III), raises the upper lid. Encircling the eye is the orbicularis oculi muscle, which is supplied by the facial nerve (CN VII). When this muscle contracts, it closes the eyelids. Eyelid Weakness Drooping of the eyelid is called ptosis. It can result from weakness of the levator muscle that elevates the upper lid in conjunction with the unopposed action of the orbicularis oculi that forcefully closes the eyelids. Weakness of the orbicularis oculi causes an open eyelid, but not ptosis. Neurologic causes of eyelid weakness include damage to the innervating cranial nerves or to the nerves’ central nuclei in the midbrain and the caudal pons. Normally, the edges of the eyelids, or palpebrae, are in such a position that the palpebral conjunctiva that lines the eyelids is not exposed and the eyelashes do not rub against the cornea. Turning in of the lid is called entropion. It is usually caused by scarring of the palpebral conjunctiva or degeneration of the fascial attachments to the lower lid that occurs with aging. Corneal irritation may occur as the eyelashes turn inward. Ectropion refers to eversion of the lower lid. The condition is usually bilateral and caused by relaxation of the orbicularis oculi muscle because of CN VII weakness or the aging process. Ectropion causes tearing and ocular irritation and may lead to inflammation of the cornea. Entropion and ectropion can be treated surgically. Electrocautery penetration of the lid conjunctiva also can be used to treat mild forms of ectropion. After electrocautery, 1294 UNIT XIII Special Sensory Function Superior rectus muscle Superior tarsal plate Tendon of superior oblique muscle Lacrimal gland Superior lacrimal duct Lacrimal sac Lateral palpebral ligament Medial palpebral ligament Inferior lacrimal duct Inferior tarsal plate Nasolacrimal duct Inferior rectus muscle Opening of duct into inferior nasal meatus Inferior oblique muscle FIGURE 54-3 The eye and its appendages: anterior view. contraction of the resulting scar tissue usually draws the lid up to its normal position. Eyelid Inflammation Blepharitis is a common bilateral inflammation of the anterior or posterior structures of eyelid margins. Anterior blepharitis involves the eyelid skin, eyelashes, and associated glands. Two main types of anterior blepharitis occur: seborrheic and staphylococcal.2,3 The seborrheic form is usually associated with seborrhea (i.e., dandruff) of the scalp or brows. Staphylococcal blepharitis may be caused by Staphylococcus epidermidis or Staphylococcus aureus, in which case the lesions are often ulcerative. The chief symptoms of anterior blepharitis are irritation, burning, redness, and itching of the eyelid margins. Treatment includes careful cleaning with a wet applicator or clean washcloth to remove the scales. When the disorder is associated with a microbial infection, an antibiotic ointment is prescribed. Posterior blepharitis is inflammation of the eyelids that involves the meibomian glands. It may result from a bacterial infection, particularly with staphylococci, or dysfunction of the meibomian glands, in which there is a strong association with acne rosacea.2,3 The meibomian glands and their orifices are inflamed, with dilation of the glands, plugging of the orifices, and abnormal secretions. The lid margins are often rolled inward to produce mild entropion, and the tears may be frothy and abnormally greasy from the meibomian secretions. Treatment of posterior blepharitis is determined by associated conjunctival and corneal changes. Long-term, low-dose systemic antibiotic therapy guided by results of bacterial cultures, along with short-term topical steroids, may be needed. A hordeolum, or stye, is caused by infection of the sebaceous glands of the eyelid and can be internal or exter- nal (Fig. 54-4A). The main symptoms are pain, redness, and swelling. The treatment is similar to that of abscesses in other parts of the body. Heat, such as a warm compress, is applied, and antibiotic ointment may be used. Incision or expression of the infectious contents of the abscess may be necessary. A chalazion is a granulomatous inflammation of a meibomian gland that may follow an internal hordeolum. It is characterized by a small nontender nodule on the upper or lower lid (see Fig. 54-4B). The conjunctiva around the chalazion is red and elevated. If the chalazion is large enough, it may press on the eyeball and distort vision. Treatment consists of surgical excision. DISORDERS OF THE LACRIMAL SYSTEM The lacrimal system includes the major lacrimal gland, which produces the tears; the puncta and tear sac, which collect the tears; and the nasolacrimal duct, which empties the tears into the nasal cavity. The lacrimal gland lies in the orbit, superior and lateral to the eyeball (see Fig. 54-3). Approximately 12 small ducts connect the lacrimal gland to the superior conjunctival fornix. Tears contain approximately 98% water, 1.5% sodium chloride, and small amounts of potassium, albumin, and glucose. The function of tears is to make a smooth optical surface by abolishing minute surface irregularities. Tears also wet and protect the delicate surface of the cornea and conjunctiva. They flush and remove irritating substances and microorganisms, and they provide the cornea with necessary nutrient substances. Tears also contain lysozymes and immunoglobulin A (IgA), IgG, and IgE, which synergistically act to protect against infection. Although IgA predominates, IgE concentrations are increased in some allergic conditions. CHAPTER 54 A Disorders of Visual Function 1295 filtrate the lacrimal and parotid glands. The disorder is associated with diminished salivary and lacrimal secretions, resulting in keratoconjunctivitis sicca (dry eye syndrome) and xerostomia (i.e., dry mouth). The syndrome occurs mainly in women near menopause and is often associated with connective tissue disorders such as rheumatoid arthritis. Persons with dry eyes complain of a dry or gritty sensation in the eye, burning, itching, inability to produce tears, photosensitivity, redness, pain, and difficulty in moving the eyelids. Dry eyes and the absence of tears can cause keratinization of the cornea and conjunctival epithelium. In severe cases, corneal ulcerations can occur. Consequent corneal scarring can cause blindness. The treatment of dry eyes includes frequent instillation of artificial tear solutions into the conjunctival sac. More prolonged duration of action can be obtained from topical preparations containing methylcellulose or polyvinyl alcohol. An ointment is useful for prolonged lubrication. Overall, these artificial tear preparations are safe and without side effects. However, the preservatives necessary to maintain their sterility can be irritating to the cornea.2,5 Dacryocystitis B FIGURE 54-4 (A) Stye (acute hordeolum). (B) Chalzion. (Bickley L.S. (2003). Bates’ guide to physical examination and history taking (8th ed., p. 178). Philadelphia: Lippincott Williams & Wilkins) Dry Eyes The thin film of tears that covers the cornea is essential in preventing drying and damage of the outer layer of the cornea. This tear film is composed of three layers: the superficial lipid layer, derived from the meibomian glands and thought to retard evaporation; the aqueous layer, secreted by the lacrimal glands; and the mucinous layer, which overlies the cornea and epithelial cells. Because the epithelial cell membranes are hydrophobic and cannot be wetted by aqueous solutions alone, the mucinous layer plays an essential role in wetting these surfaces. Periodic blinking of the eyes is needed to maintain a continuous tear film over the ocular surface. Several conditions reduce the functioning of the lacrimal glands. With aging, the lacrimal glands diminish their secretion, and as a result, many older persons awaken from a night’s sleep with highly irritated eyes. Dry eyes also result from loss of reflex lacrimal gland secretion because of congenital defects, infection, irradiation, damage to the parasympathetic innervation of the gland, and medications such as antihistamines and drugs with an anticholinergic action. Wearing contact lenses contributes to eye dryness through decreased blinking. Sjögren’s syndrome is a systemic disorder in which lymphocytes and plasma cells in- Dacryocystitis is an infection of the lacrimal sac. It occurs most often in infants and in persons older than 54 years of age.2 It is usually unilateral and most often occurs secondary to obstruction of the nasolacrimal duct. Often, the cause of the obstruction is unknown, although there may be a history of severe trauma to the midface. The symptoms include tearing and discharge, pain, swelling, and tenderness. The treatment includes application of warm compresses and antibiotic therapy. In chronic forms of the disorder, surgical repair of the tear duct may be necessary. In infants, dacryocystitis is usually caused by failure of the nasolacrimal ducts to open spontaneously before birth. When a duct fails to open, a secondary dacryocystitis may develop. These infants are usually treated with gentle massage of the tear sac, instillation of antibiotic drops into the conjunctival sac, and, if that fails, probing of the tear duct. In summary, the eyelids serve to protect the eye. Ptosis refers to drooping of the upper lid, which is caused by injury to CN III. Entropion, which refers to turning in the upper eyelid and eyelashes, is discomforting and causes corneal irritation. Ectropion, or eversion of the lower eyelid, causes tearing and may lead to corneal inflammation. Marginal blepharitis is the most common disorder of the eyelids. It commonly is caused by a staphylococcal infection or seborrhea (i.e., dandruff). The lacrimal system includes the major lacrimal gland, which produces the tears, the puncta and tear sac, which collect the tears, and the nasolacrimal duct, which empties the tears into the nasal cavity. Tears protect the cornea from drying and irritation. Impaired tear production or conditions that prevent blinking and the spread of tears produce drying of the eyes and predispose them to corneal irritation and injury. Dacryocystitis is an infection of the lacrimal sac. 1296 UNIT XIII Special Sensory Function Disorders of the Conjunctiva, Cornea, and Uveal Tract After completing this section of the chapter, you should be able to meet the following objectives: ✦ Compare symptoms associated with red eye caused by conjunctivitis, corneal irritation, and acute glaucoma ✦ List at least four causes of red eye ✦ Describe the appearance of corneal edema ✦ Characterize the manifestations, treatment, and possible complications of bacterial, Acanthamoeba, and herpes keratitis ✦ Describe the structures of the uveal tract ✦ Describe tests used in assessing the pupillary reflex and cite the possible causes of abnormal pupillary reflexes DISORDERS OF THE CONJUNCTIVA The conjunctiva is a thin layer of mucous membrane that lines the anterior surface of both eyelids as the palpebral conjunctiva and folds back over the anterior surface of the optic globe as the ocular or bulbar conjunctiva. The ocular conjunctiva covers only the sclera or white portion of the optic globe, not the cornea. When both eyes are closed, the conjunctiva lines the closed conjunctival sac. Although the conjunctiva protects the eye by preventing foreign objects from entering beyond the conjunctival sac, its main function is the production of a lubricating mucus that bathes the eye and keeps it moist. Conjunctivitis, or inflammation of the conjunctiva (i.e., red eye or pink eye), is one of the most common forms of eye disease.4,5 It may result from bacterial or viral infection, allergens, chemical agents, physical irritants, or radiant energy. Infections may extend from areas adjacent to the conjunctiva or may be blood-borne, such as in measles or chickenpox. Newborns can contract conjunctivitis during the birth process. Infectious forms of conjunctivitis are often bilateral and may involve other family members and close associates. Unilateral disease suggests sources of irritation such as foreign bodies or chemical irritation. Depending on the cause, conjunctivitis can vary in severity from a mild hyperemia (redness) with tearing to severe conjunctivitis with purulent drainage. The conjunctiva is extremely sensitive to irritation and inflammation. Important symptoms of conjunctivitis are a foreign body sensation, a scratching or burning sensation, itching, and photophobia. Severe pain suggests corneal rather than conjunctival disease. Itching is common in allergic conditions. A discharge, or exudate, may be present with all types of conjunctivitis and may cause transient blurring of vision. It is usually watery when the conjunctivitis is caused by allergy, a foreign body, or viral infection and mucopurulent in the presence of bacterial or fungal infection. A characteristic of many forms of conjunctivitis is papillary hypertrophy. This occurs because the palpebral conjunctiva is bound to the tarsus by fine fibrils. As a result, inflammation that develops between the fibrils causes the conjunctiva to be elevated in mounds called papillae. When the papillae are small, the conjunctiva has a smooth, velvety appearance. Red papillary conjunctivitis suggests bacterial or chlamydial conjunctivitis. In allergic conjunctivitis, the papillae often become flat topped, polygonal, and milky in color and have a cobblestone appearance. The diagnosis of conjunctivitis is based on history, physical examination, and microscopic and culture studies to identify the cause. Because a red eye may be the sign of several eye conditions, it is important to differentiate between redness caused by conjunctivitis and that caused by more serious eye disorders, such as corneal lesions and acute glaucoma. In contrast to corneal lesions and acute glaucoma, conjunctivitis produces injection (i.e., enlargement and redness) of the peripheral conjunctival blood vessels rather than those radiating around the corneal limbus. Conjunctivitis also produces only mild discomfort, as compared with the moderate to severe discomfort associated with corneal lesions or the severe and deep pain associated with acute glaucoma. Infectious forms of conjunctivitis are usually bilateral and may involve other family members and associates. Unilateral disease suggests sources of irritation such as foreign bodies or chemical irritation. Allergic Conjunctivitis Allergic conjunctivitis encompasses a spectrum of conjunctival conditions usually characterized by itching. The most common of these is seasonal allergic rhinoconjunctivitis, or hay fever. Seasonal allergic conjunctivitis is an IgE-mediated hypersensitivity reaction precipitated by small airborne allergens such as pollens.6 It typically causes bilateral tearing, itching, and redness of the eyes. The treatment of seasonal allergic rhinoconjunctivitis includes allergen avoidance, the use of cold compresses, oral antihistamines, and vasoconstrictor eye drops. Allergic conjunctivitis also has been successfully treated with topical mast cell stabilizers, histamine H1 receptor antagonists, and topical nonsteroidal antiinflammatory drugs.6 All three types of agents are well tolerated and have a rapid onset of action. In severe cases, a short course of topical corticosteroids may be required to afford symptomatic relief. Infectious Conjunctivitis The agents of infectious conjunctivitis include bacteria, viruses, and chlamydiae. Infections may spread from areas adjacent to the conjunctiva or may be blood-borne, such as in measles or chickenpox. Newborns can contract conjunctivitis during the birth process. Bacterial Conjunctivitis. Bacterial conjunctivitis may present as a hyperacute, acute, or chronic infection. Hyperacute conjunctivitis is a severe, sight-threatening ocular infection. The infection has an abrupt onset and is characterized by a copious amount of yellow-green drainage. The symptoms, which typically are progressive, include conjunctival redness and chemosis, lid swelling, and tender, swollen preaurical lymph nodes. The most common causes of hyperacute purulent conjunctivitis are Neisseria gonorrhoeae and Neisseria meningitidis, with N. gonorrhoeae being the most common.3–5 Gonococcal ocular infections left untreated result in corneal ulceration with ultimate perforation, and CHAPTER 54 sometimes permanent loss of vision. Diagnostic methods include immediate Gram staining of ocular specimens and special cultures for Neisseria species. Treatment includes systemic antibiotics supplemented with ocular antibiotics. Because of the increasing prevalence of penicillin-resistant N. gonorrhoeae, antibiotic choice should be determined by current information regarding antibiotic sensitivity. Acute bacterial conjunctivitis typically presents with burning, tearing, and mucopurulent or purulent discharge. Common agents of bacterial conjunctivitis are Streptococcus pneumoniae, S. aureus, and Haemophilus influenzae.4 The eyelids are sticky, with possible excoriation of the lid margins. Treatment may include local application of antibiotics. The disorder is usually self-limited, lasting approximately 10 to 14 days if untreated. Scrupulous eyelid hygiene and prompt adequate treatment of infected persons and their contacts are effective. Chronic bacterial conjunctivitis most commonly is caused by Staphylococcus species, although other bacteria may be involved. It is often associated with blepharitis and bacterial colonization of eyelid margins. The symptoms of chronic bacterial conjunctivitis vary and can include itching, burning, foreign body sensation, and morning eyelash crusting. Other symptoms include flaky debris and erythema along the lid margins, eyelash loss, and eye redness. Some people with chronic bacterial conjunctivitis also have recurrent styes and chalazia of the lid margins. Treatment includes good eyelid hygiene and application of topical antibiotics. Viral Conjunctivitis. Etiologic agents of viral conjunctivitis include adenoviruses, herpesviruses, and enteroviruses. Adenovirus type 3 infection is usually associated with pharyngitis, fever, and malaise.4,5 It causes generalized hyperemia, copious tearing, and minimal exudate. Children are affected more often than adults. Swimming pools contaminated because of inadequate chlorination are common sources of infection. Infections due to adenovirus types 4 and 7 are often associated with acute respiratory disease. These viruses are rapidly disseminated when large groups mingle with infected individuals (e.g., military recruits). Adenovirus type 8 epidemics are associated with contamination of ophthalmic products and equipment. No specific treatment for this type of viral conjunctivitis exists; it usually lasts 7 to 14 days. Preventive measures include scrupulous eyelid hygiene and avoiding shared use of eyedroppers, eye makeup, goggles, and towels. Herpes simplex virus conjunctivitis is characterized by unilateral infection, irritation, mucoid discharge, pain, and mild photophobia. Herpetic vesicles may develop on the eyelids and lid margins. Although the infection is usually caused by the type 1 herpesvirus, it also can be caused by the type 2 virus that causes genital herpes. It is often associated with herpes simplex virus keratitis, in which the cornea shows discrete epithelial lesions. Treatment involves the use of systemic or local antiviral agents. Topical antiviral agents such as vidarabine, trifluridine, or idoxuridine usually provide prompt relief.4 Local corticosteroid preparations increase the activity of the herpes simplex virus, apparently by enhancing the destructive effect of Disorders of Visual Function 1297 collagenase on the collagen of the cornea. The use of these medications should be avoided in those suspected of having herpes simplex conjunctivitis or keratitis. Chlamydial Conjunctivitis. Chlamydial conjunctivitis is usually a benign suppurative conjunctivitis transmitted by the type of Chlamydia trachomatis (serotypes D through K) that causes venereal infections (see Chapter 48). It is spread by contaminated genital secretions and occurs in newborns of mothers with C. trachomatis infections of the birth canal. It also can be contracted through swimming in unchlorinated pools. The incubation period varies from 5 to 12 days, and the disease may last for several months if untreated. The infection is usually treated with appropriate oral antibiotics. A more serious form of infection is caused by a different strain of C. trachomatis (serotypes A through C). This form of chlamydial infection affects the conjunctiva and causes ulceration and scarring of the cornea. It is the leading cause of preventable blindness in the world. Although the agent is widespread, it is seen mostly in developing countries, particularly those of Africa, Asia, and the Middle East.7 It is transmitted by direct human contact, contaminated objects (fomites), and flies. Ophthalmia Neonatorum Ophthalmia neonatorum is a form of conjunctivitis that occurs in newborns younger than 1 month of age and is usually contracted during or soon after vaginal delivery. Many causes are known, including N. gonorrhoeae, Pseudomonas, and C. trachomatis.8 Epidemiologically, these infections reflect those sexually transmitted diseases most common in a particular area. Once the most common form of conjunctivitis in the newborn, gonococcal ophthalmia neonatorum has an incidence of 0.3% of live births in the United States; C. trachomatis has an incidence of 8.2% of live births.8 Drops of 0.5% erythromycin or 1% silver nitrate are applied immediately after birth to prevent gonococcal ophthalmia. Silver nitrate instillation may cause mild, self-limited conjunctivitis. Signs of ophthalmia neonatorum include redness and swelling of the conjunctiva, swelling of the eyelids, and discharge, which may be purulent. The conjunctivitis caused by silver nitrate occurs within 6 to 12 hours of birth and clears within 24 to 48 hours.8 The incubation period for N. gonorrhoeae is 2 to 5 days and for C. trachomatis, 5 to 14 days. Infection should be suspected when conjunctivitis develops 48 hours after birth.7 Ophthalmia neonatorum is a potentially blinding condition, and it can cause serious and potentially systemic manifestations. It requires immediate diagnosis and treatment. DISORDERS OF THE CORNEA At the anterior part of the eyeball, the outer covering of the eye is modified to form the transparent cornea, which bulges anteriorly from its junction with the sclera (Fig. 54-5). A major part of the refraction (i.e., bending) of 1298 UNIT XIII Special Sensory Function Epithelium Bowman’s membrane Cornea Stroma Descemet’s membrane Trabecular meshwork Canal of Schlemm Endothelium Conjunctiva Ciliary process Sphincter muscle Iris Pigment layer Ciliary muscle Dilatory muscle Sclera Lens Zonular fibers Ciliary epithelium FIGURE 54-5 Anterior chamber angle and surrounding structures. Ora serrata light rays and focusing of vision occurs in the cornea. Three layers of tissue form the cornea: an extremely thin outer epithelial layer, which is continuous with the bulbar conjunctiva; a middle layer called the substantia propria or stroma; and an inner endothelial layer, which lies next to the aqueous humor of the anterior chamber. The substantia propria is composed of regularly arranged collagen bundles embedded in a mucopolysaccharide matrix. This organization of the collagen fibers, which makes the substantia propria transparent, is necessary for light transmission. Hydration within a limited range is necessary to maintain the spacing of the collagen fibers and transparency. The cornea is avascular and obtains its nutrient and oxygen supply by diffusion from blood vessels of the adjacent sclera, from the aqueous humor at its deep surface, and from tears. The corneal epithelium is heavily innervated by sensory neurons (trigeminal nerve [CN V], ophthalmic division [CN V1]). Epithelial damage causes discomfort that ranges from a foreign body sensation and burning of the eyes to severe, stabbing or knifelike, incapacitating pain. Reflex lacrimation is common. Disorders of the cornea include trauma and infections, abnormal corneal deposits, and arcus senilis. Corneal Trauma Trauma that causes abrasions of the cornea can be extremely painful, but if minor, the abrasions usually heal in a few days. The epithelial layer can regenerate, and small defects heal without scarring. If the stroma is damaged, healing occurs more slowly, and the danger of infection is increased. Injuries to Bowman’s membrane and the stro- mal layer heal with scar formation and permanent opacification. Opacities of the cornea impair the transmission of light. A minor scar can severely distort vision because it disturbs the refractive surface. The integrity of the epithelium and the endothelium is necessary to maintain hydration of the cornea within a limited range. Damage to either structure leads to edema and loss of transparency. Among the causes of corneal edema is the prolonged and uninterrupted wearing of hard contact lenses, which can deprive the epithelium of oxygen, disrupting its integrity. The edema disappears spontaneously when the cornea contacts the atmosphere. Corneal edema also occurs after a sudden rise in intraocular pressure. With corneal edema, the cornea appears dull, uneven, and hazy. Visual acuity decreases, and iridescent vision (i.e., rainbows around lights) occurs. Iridescent vision results from epithelial and subepithelial edema, which splits white light into its component parts, with blue in the center and red on the outside. Keratitis Keratitis refers to inflammation of the cornea. It can be caused by infections, hypersensitivity reactions, ischemia, defects in tearing, trauma, and interruption in sensory innervation, as occurs with local anesthesia. Scar tissue formation due to keratitis is the leading cause of blindness and impaired vision throughout the world. Most of this vision loss is preventable if the condition is diagnosed early and appropriate treatment is begun. Diagnosis of keratitis is based on history of trauma, medication use, and signs and symptoms associated with CHAPTER 54 corneal irritation and disease.9 Because the cornea has many pain fibers, superficial or deep corneal abrasions cause discomfort, photophobia, and lacrimation. The discomfort may range from a foreign body sensation to severe pain. Defective vision results from the changes in transparency and curvature of the cornea that occur. Because of the discomfort involved, examination of the eye is often eased by instillation of a local anesthetic agent. Fluorescein staining can be used to outline an ulcerated area. The biomicroscope (slit lamp) is used for proper examination of the cornea. In cases of an infectious etiology, scrapings from the ulcer are obtained for staining and culture studies. Keratitis can be divided into two types: nonulcerative, in which all the layers of the epithelium are affected but the epithelium remains intact, and ulcerative, in which parts of the epithelium, stroma, or both are destroyed. Nonulcerative or interstitial keratitis is associated with many diseases, including syphilis, tuberculosis, and lupus erythematosus. It also may result from a viral infection entering through a small defect in the cornea. Treatment is usually symptomatic. Causes of ulcerative keratitis include infectious agents such as those causing conjunctivitis (e.g., Staphylococcus, S. pneumoniae, Chlamydia), exposure trauma, and use of extended-wear contact lenses. Bacterial keratitis is aggressive and demands immediate care. Exposure trauma may result from deformities of the lid, paralysis of the lid muscles, or severe exophthalmos. Mooren’s ulcer is a chronic, painful, indolent ulcer that occurs in the absence of infection. It is usually seen in older persons and may affect both eyes. Although the cause is unknown, an autoimmune origin is suspected. Acanthamoeba is a free-living protozoan that thrives in contaminated water. Acanthamoeba keratitis is an increasingly serious and sight-threatening complication of wearing a soft contact lens, particularly when homemade saline solutions are used for cleaning.9 It also may occur in non–contact lens wearers after exposure to contaminated water or soil. It is characterized by pain that is disproportionate to the clinical manifestations, redness of the eye, and photophobia. The disorder commonly is misdiagnosed as herpes keratitis. Diagnosis is confirmed by scrapings and culture with specially prepared medium. In the early stages of infection, epithelial débridement may be beneficial. Treatment includes intensive use of topical antibiotics. However, the organism may encyst within the corneal stroma, making treatment more difficult. Keratoplasty may be necessary in advanced disease to arrest the progression of the infection. Herpes Simplex Keratitis. Herpes simplex virus (HSV) keratitis is the most common cause of corneal ulceration in the United States. Most cases are caused by HSV type 1 infections. However, in neonatal infections acquired during passage through the birth canal, approximately 80% are caused by HSV type 2. The disease can occur as a primary or recurrent infection.9 Primary infections cause follicular conjunctivitis and blepharitis, characterized by a rounded cobblestone pattern of avascular lesions. Epithelial keratitis may develop. After the initial primary infection, the virus Disorders of Visual Function 1299 may persist in a quiescent or latent state that remains in the trigeminal ganglion and possibly in the cornea without causing signs of infection. During childhood, mild primary herpes simplex virus infection may go unnoticed. Recurrent infection may be precipitated by various poorly understood, stress-related factors that reactivate the virus. Involvement is usually unilateral. The first symptoms are irritation, photophobia, and tearing. Some reduction in vision may occur when the lesion affects the central part of the cornea. Because corneal anesthesia occurs early in the disease, the symptoms may be minimal, and the person may delay seeking medical care. A history of fever blisters or other herpetic infection is often noted, but corneal lesions may be the only sign of recurrent herpes infection. Most typically, the corneal lesion involves the epithelium and has a typical branching pattern. These epithelial lesions heal without scarring. Herpetic lesions that involve the stromal layer of the cornea produce increasingly severe corneal opacities. They are thought to have an immune rather than an infectious cause. The most common cause of corneal blindness in the Western world is stromal scarring from herpes simplex keratitis.9 The treatment of HSV keratitis focuses on eliminating viral replication within the cornea while minimizing the damaging effects of the inflammatory process. It involves the use of epithelial debridement and drug therapy. Debridement is used to remove the virus from the corneal epithelium. Topical antiviral agents such as trifluridine (Viroptic) drops, idoxuridine (IDU) drops, or vidarabine (Vira-A) ointment are used to promote healing. Corticosteroid drugs increase viral replication. With a few exceptions, their use is usually contraindicated. Varicella Zoster Ophthalmicus. Herpes zoster or shingles is a relatively common infection caused by herpesvirus type 3, the same virus that causes varicella (chickenpox).10 It occurs when the varicella virus, which has remained dormant in the neurosensory ganglia since the primary infection, is reactivated. Herpes ophthalmicus, which represents 10% to 25% of all cases of herpes zoster, occurs when reactivation of the latent virus occurs in the ganglia of the ophthalmic division of the trigeminal nerve.9,10 Immunocompromised persons, particularly those with human immunodeficiency disease (HIV) infection, are at higher risk for developing herpes zoster ophthalmicus than those with a normally functioning immune system. Herpes zoster ophthalmicus usually presents with malaise, fever, headache, and burning and itching of the periorbital area. These symptoms commonly precede the eruption by 1 or 2 days. The rash, which is initially vesicular, becomes pustular and then crusting (see Chapter 61). Involvement of the tip of the nose and lid margins indicates a high likelihood of ocular involvement. Ocular signs include conjunctivitis, keratitis, and anterior uveitis, often with elevated intraocular pressure. Persons with corneal disease present with varying degrees of decreased vision, pain, and light insensitivity. Treatment includes the use of high-dose oral antiviral drugs (acyclovir, valacyclovir). Initiation of treatment 1300 UNIT XIII Special Sensory Function within the first 72 hours after the appearance of the rash reduces the incidence of ocular complications but not the postherpetic neuralgia (see Chapter 50). Abnormal Corneal Deposits The cornea frequently is the site for deposition of abnormal metabolic products. In hypercalcemia, calcium salts can precipitate in the cornea, producing a cloudy band keratopathy. Cystine crystals are deposited in cystinosis, cholesterol esters in hypercholesterolemia, and a golden ring of copper (i.e., Kayser-Fleischer ring) in hepatolenticular degeneration due to Wilson’s disease. Pharmacologic agents, such as chloroquine, can result in crystal deposits in the cornea. Arcus senilis is an extremely common, bilateral, benign corneal degeneration that may occur at any age but is more common in the elderly. It consists of a grayish-white infiltrate, approximately 2 mm wide, that occurs at the periphery of the cornea. It represents an extracellular lipid infiltration and commonly is associated with hyperlipidemia. Arcus senilis does not produce visual symptoms, and there is no treatment for the disorder. Corneal Transplantation Advances in ophthalmologic surgery permit corneal transplantation using a cadaver cornea. Unlike kidney or heart transplantation procedures, which are associated with considerable risk for rejection of the transplanted organ, the use of cadaver corneas entails minimal danger of rejection because this tissue is not exposed to the vascular system and therefore the immunologic defense system. Instead, the success of this type of transplantation operation depends on the prevention of scar tissue formation, which would limit the transparency of the transplanted cornea. DISORDERS OF THE UVEAL TRACT The middle vascular layer, or uveal tract, is an incomplete ball with gaps at the pupil and the optic nerve (see Fig. 54-3). The pigmented uveal tract has three distinct regions: the choroid, ciliary body, and iris. The choroid is a highly vascular, dark-brown membrane that forms the posterior five sixths of the uveal tract. Its blood vessels provide nourishment for the other layers of the eyeball. Its brown pigment, produced by melanocytes, absorbs light within the eyeball and light that penetrates the retina. The light absorptive function prevents the scattering of light and is important for visual acuity, particularly with high background illumination levels. The ciliary body is a thickened ring of tissue that encircles the lens. It has smooth muscle and secretory functions. Its smooth muscle function contributes to changes in lens shape and its secretory function to the production of aqueous humor. The iris is an adjustable diaphragm that permits changes in pupil size and in the light entering the eye. The posterior surface of the iris is formed by a two-layer epithelium continuous with those layers covering the ciliary body. The anterior layer contains the dilator or radial muscles of the iris. Just anterior to these muscles is a layer of highly vascular connective tissue. Embedded in this layer are concentric rings of smooth muscle that compose the sphincter muscle of the pupil. The anterior layer of the iris forms an irregular anterior surface, containing many fibroblasts and melanocytes. Eye color differences result from the density of the pigment. The amount of pigment decreases from that found in dark brown eyes through shades of brown and gray to that found in blue eyes. Several mutations affect the pigment of the uveal tract, including albinism. Albinism is a genetic (autosomal recessive trait) deficiency of tyrosinase, the enzyme needed for the synthesis of melanin by the melanocytes. Tyrosinasenegative albinism, also called classic albinism, is characterized by an absence of tyrosinase; affected persons have white hair, pink skin, and light blue eyes. In these persons, excessive light penetrates the unpigmented iris and choroid, and, to some extent, the anterior sclera. Their photoreceptors are flooded with excess light, and visual acuity is markedly reduced. Excess stimulation of the photoreceptors at normal or high illumination levels is experienced as painful photophobia. Uveitis Inflammation of the entire uveal tract, which supports the lens and neural components of the eye, is called uveitis. It is one of several inflammatory disorders of ocular tissue with clinical features in common and an immunologically based cause.5 A serious consequence of uveitis can be the involvement of the underlying retina. Parasitic invasion of the choroid can result in local atrophic changes that usually involve the retina; examples include toxoplasmosis and histoplasmosis. Sarcoid deposition in the form of small nodules results in irregularities of the underlying retinal surface. THE PUPIL AND PUPILLARY REFLEXES Changes in pupil size are controlled by contraction or relaxation of the dilator sphincter muscle of the iris. The pupillary reflex, which controls the size of the pupillary opening, is controlled by the autonomic nervous system, with the parasympathetic nervous system producing pupillary constriction or miosis and the sympathetic nervous system producing pupillary dilation or mydriasis. The sphincter muscle that produces pupillary constriction is innervated by postganglionic parasympathetic neurons of the ciliary ganglion and other scattered ganglion cells between the scleral and choroid layers. Part of the oculomotor (CN III) nucleus is called the Edinger-Westphal nucleus.11 This autonomic nucleus, found in the midbrain, provides the preganglionic innervation for these parasympathetic axons. Pupillary dilation is provided by sympathetic innervation under excitatory descending control from the hypothalamus. Innervation is derived from preganglionic neurons in the upper thoracic cord, which send axons along the sympathetic chain to synapse with postganglionic neurons in the superior cervical ganglion. Postganglionic fibers travel along the surfaces of the carotid and smaller arteries to reach the eye. The pupillary reflex is controlled by a region in the midbrain called the pretectum. Pretectal areas on each side CHAPTER 54 of the brain are connected, explaining the binocular aspect of the light reflex. The afferent stimuli for pupillary constriction arise in the ganglionic cells of the retina and are transmitted to the pretectal nuclei at the junction of the thalamus and the midbrain, and from there to preganglionic neurons in the oculomotor (CN III) nuclei (Fig. 54-6). Normal function of the pupillary reflex mechanism can be tested by shining a penlight into one eye of the person being tested. To avoid a change in pupil size due to accommodation, the person is asked to stare into the distance. A rapid constriction of the pupil exposed to light should occur; this is called the direct pupillary light reflex. Because the reflex is normally bilateral, the contralateral pupil also should constrict, a reaction called the consensual pupillary light reflex. The circuitry of the light reflex is partially separated from the main visual pathway. This is illustrated by the fact that the pupillary reflex remains unaffected when lesions to the optic radiations or the visual cortex occur. The cortically blind person retains direct and consensual light reflexes. Integrity of the dual autonomic control of pupillary diameter is vulnerable to trauma, tumor enlargement, or vascular disease. With diffuse damage to the forebrain involving the thalamus and hypothalamus, the pupils are typically small but respond to light. Damage to the CN III nucleus or nerve eliminates innervation of four of the six extraocular muscles and the levator muscle of the upper lid, and it results in permanent pupillary dilation in the af- Optic nerve Ciliary ganglion Red nucleus Optic tract Edinger-Westphal nucleus IIIn Disorders of Visual Function 1301 fected eye. Lesions affecting the cervical spinal cord or the ascending sympathetic ganglionic chain in the neck or internal carotid artery (e.g., Horner’s syndrome) can interrupt the sympathetic control of the iris dilator muscle, resulting in permanent pupillary constriction. Tumors of the orbit that compress structures behind the eye can eliminate all pupillary reflexes, usually before destroying the optic nerve. Pupillary size can also be differentially affected by pharmacologic agents. Bilateral pupillary constriction is characteristic of opiate usage. Pupillary dilation results when topical parasympathetic blocking agents such as atropine are applied and sympathetic pupillodilatory function is left unopposed. These medications are used by ophthalmologists to facilitate the examination of the transparent media and fundus of the eye. Miotic drugs (e.g., pilocarpine), which are used in the treatment of narrowangle glaucoma, produce pupil constriction and in that manner facilitate aqueous humor circulation. In summary, the conjunctiva lines the inner surface of the eyelids and covers the optic globe to the junction of the cornea and sclera. Conjunctivitis, also called red eye or pink eye, may result from bacterial or viral infection, allergens, chemical agents, physical agents, or radiant energy. It is important to differentiate between redness caused by conjunctivitis and that caused by more serious eye disorders, such as acute glaucoma or corneal lesions. Keratitis, or inflammation of the cornea, can be caused by infections, hypersensitivity reactions, ischemia, trauma, or defects in tearing. Trauma or disease that involves the stromal layer of the cornea heals with scar formation and permanent opacification. These opacities interfere with the transmission of light and may impair vision. The uveal tract is the middle vascular layer of the eye. It contains melanocytes that prevent diffusion of light through the wall of the optic globe. Inflammation of the uveal tract (uveitis) can affect visual acuity. The pupillary reflex, which controls the size of the pupil, is controlled by the autonomic nervous system. The parasympathetic nervous system controls pupillary constriction, and the sympathetic nervous system controls pupillary dilation. Lateral geniculate nucleus Intraocular Pressure and Glaucoma After completing this section of the chapter, you should be able to meet the following objectives: ✦ Describe the formation and outflow of aqueous humor Pretecto-oculomotor tract Posterior commissure Pulvinar from the eye and relate to the development of glaucoma ✦ Compare closed-angle and open-angle glaucoma ✦ Explain why glaucoma leads to blindness Pretectal nucleus FIGURE 54-6 Diagram of the path of the pupillary light reflex. (Reproduced with permission from Walsh F.B., Hoyt W.F. [1969]. Clinical neuro-ophthalmology [3rd ed., vol. 1]. Baltimore: Williams & Wilkins) Glaucoma includes a group of conditions that produce an elevation in intraocular pressure. If left untreated, the pressure may increase sufficiently to cause ischemia and degeneration of the optic nerve, leading to progressive 1302 UNIT XIII Special Sensory Function blindness. An estimated 60 million people worldwide currently have glaucoma. About 6 million people worldwide are blind from glaucoma, including 100,000 Americans, making it the leading cause of blindness in the United States.12 African Americans are three to four times more likely to have open-angle glaucoma as whites, and even greater racial disparities exist in terms of blindness from the disease. The condition is often asymptomatic, and a significant loss of peripheral vision may occur before medical attention is sought. CONTROL OF INTRAOCULAR PRESSURE The intraocular pressure is largely regulated by the aqueous humor, which occupies the anterior segment of the optic globe. The aqueous humor serves to maintain the intraocular pressure and provides for the nutritive needs of the lens and posterior cornea. It contains a low concentration of protein and high concentrations of ascorbic acid, glucose, and amino acids. It also mediates the exchange of respiratory gases. The aqueous humor is produced by the ciliary epithelium in the posterior chamber and flows between the anterior surface of the lens and posterior surface of the iris, through the pupil and into the anterior chamber. Aqueous humor, which is an ultrafiltrate of plasma, is secreted at an average rate of 2 to 3 microliters per minute.13 Essentially all of the aqueous humor is secreted by the ciliary processes, which are linear folds that project from the ciliary body into the space behind the iris where the lens ligaments and ciliary muscle attach to the eyeball. Aqueous humor leaves through the iridocorneal angle between the iris and the sclera. Here it filters through the trabecular meshwork and enters the canal of Schlemm for return to the venous circulation (see Fig. 54-5). The canal of Schlemm is actually a thin-walled vein that extends circumferentially around the eye. Its endothelial membrane is so porous that even large protein molecules up to the size of a red blood cell can pass from the posterior chamber into the canal of Schlemm. The interior pressure of the eye must exceed atmospheric pressure to prevent the eyeball from collapsing. Hydrostatic pressure of the aqueous humor results from a balance of several factors: the rate of secretion, the resistance to flow between the iris and the ciliary body, and the resistance to resorption at the trabeculated region of the sclera at the iridocorneal angle. Normally, the rate of aqueous production is equal to the rate of aqueous outflow, and the intraocular pressure is maintained within a normal range of 9 to 21 mm Hg. However, data suggest that the value may be closer to 12 ±1 mm Hg in young, healthy adults during daylight hours. The mean value increases by approximately 1 mm Hg per decade after 40 years of age.14 GLAUCOMA Glaucoma is an optic neuropathy characterized by optic disk cupping and visual field loss. It usually results from an increase in intraocular pressure that results from abnor- malities in the balance between aqueous production and outflow. The most common cause is an interference with aqueous outflow from the anterior chamber, rather than overproduction of aqueous humor. Glaucoma is commonly classified as angle-closure (i.e., narrow-angle) or open-angle (i.e., wide-angle) glaucoma, depending on the location of the compromised aqueous humor circulation. Glaucoma may occur as a congenital or an acquired condition, and it may manifest as a primary or secondary disorder. Primary glaucoma occurs without evidence of preexisting ocular or systemic disease. Secondary glaucoma can result from inflammatory processes that affect the eye, from tumors, or from the blood cells of traumaproduced hemorrhage that obstruct the outflow of aqueous humor. In glaucoma, temporary or permanent impairment of vision results from pressure-induced degenerative changes in the retina and optic nerve and from corneal edema and opacification. Damage to optic nerve axons in the region of the optic nerve can be recognized on ophthalmoscopic examination. The normal optic disk has a central depression called the optic cup. With progressive atrophy of axons caused by increased intraocular pressure, pallor of the optic disk develops, and the size and depth of the optic cup increase. Because changes in the optic cup precede the visual field loss, regular ophthalmoscopic examination is important for detecting eye changes that occur with increased intraocular pressure. Stereoscopic viewing during slit-lamp examination improves the accuracy of evaluation.15 Advances in computer technology allow detection and quantification of visual changes due to glaucoma. These tests of vision include color vision analysis, blueon-yellow visual field testing and testing of contrast sensitivity, dark adaptation, and other tests of retinal function. In the future, these tests are likely to be used in detecting visual field defects not currently detected by standard means. Angle-Closure Glaucoma Angle-closure glaucoma results from occlusion of the anterior chamber angle by the iris (Fig. 54-7). It is most likely to develop in eyes with preexisting narrow anterior chambers. An acute attack is often precipitated by pupillary dilation, which causes the iris to thicken, blocking the circulation between the posterior and anterior chambers.5,12,15 Approximately 5% to 10% of all cases of glaucoma fall into this category. Angle-closure glaucoma usually occurs as the result of an inherited anatomic defect that causes a shallow anterior chamber. It is seen more commonly in people of Far-Eastern, Asian, or Inuit (Eskimo) descent and in people with hypermetropic eyes.12,15 This defect is exaggerated by the anterior displacement of the peripheral iris that occurs in older persons because of the increase in lens size that occurs with aging. The depth of the anterior chamber can be evaluated by transillumination or by a technique called gonioscopy. Gonioscopy uses a special contact lens and mirrors or prisms to view and measure the angle of the anterior chamber. The transillumination method uses only a penlight. The light source is held at the temporal side of the CHAPTER 54 Iridocorneal angle Anterior chamber Posterior chamber FIGURE 54-7 Narrow anterior chamber. eye and directed horizontally across the iris. In persons with a normal-sized anterior chamber, the light passes through the chamber to illuminate both halves of the iris. In persons with a narrow anterior chamber, only the half of the iris adjacent to the light source is illuminated. Symptoms of acute angle-closure glaucoma are related to sudden, intermittent increases in intraocular pressure. These occur after prolonged periods in the dark, emotional upset, and other conditions that cause extensive and prolonged dilation of the pupil. Administration of pharmacologic agents such as atropine that cause pupillary dilation (mydriasis) also can precipitate an acute episode of increased intraocular pressure in persons with the potential for angle-closure glaucoma. Attacks of increased intraocular pressure are manifested by ocular pain and blurred or iridescent vision caused by corneal edema.12 The pupil may be enlarged and fixed. Symptoms are often spontaneously relieved by sleep and conditions that promote pupillary constriction. With repeated or prolonged attacks, the eye becomes reddened, and edema of the cornea may develop, giving the eye a hazy appearance. A unilateral, often excruciating, headache is common. Nausea and vomiting may occur, causing the headache to be confused with migraine. Some persons with congenitally narrow anterior chambers never develop symptoms, and others develop symptoms only when they are elderly. Because of the dangers of vision loss, those with narrow anterior chambers should be warned about the significance of blurred vision, halos, and ocular pain. Sometimes, decreased visual acuity and an unreactive pupil may be the only clue to angle-closure glaucoma in the elderly. The treatment of acute angle-closure glaucoma is primarily surgical. It involves creating an opening between the anterior and posterior chambers with laser or incisional iridectomy to allow aqueous humor to bypass the pupillary block. The anatomic abnormalities responsible for angle-closure glaucoma are usually bilateral, and prophylactic surgery is often performed on the other eye. Disorders of Visual Function 1303 Open-Angle Glaucoma Primary open-angle glaucoma is the most common form of glaucoma. It tends to manifest after 35 years of age, with an incidence of 0.5% to 2% among persons 40 years of age and older. The condition is characterized by an abnormal increase in intraocular pressure that occurs without obstruction at the iridocorneal angle, hence the name openangle glaucoma. Instead, it usually occurs because of an abnormality of the trabecular meshwork that controls the flow of aqueous humor into the canal of Schlemm.12,16 Primary open-angle glaucoma is usually asymptomatic and chronic, causing progressive damage to the optic nerve and visual field loss unless it is appropriately treated. Elevated intraocular pressure is the major risk factor for open-angle glaucoma, but it is not the only diagnostic factor. Some people maintain a higher intraocular pressure without evidence of optic nerve or visual field loss. It is suggested that these people be described as glaucoma suspects or ocular hypertensives.15 The etiology of primary open-angle glaucoma remains unclear. There is question as to whether damage to the optic nerve is the result of excessive intraocular pressure, decreased blood flow to the optic nerve, or both factors. The only known causative risk factors are elevated intraocular pressure and insults to the eye, including trauma, uveitis, and corticosteroid therapy. Risk factors for this disorder include: an age of 40 years and older, black race, a positive first-degree family history, diabetes mellitus, and myopia.15 In some persons, the use of moderate amounts of topical or inhaled corticosteroid medications can cause an increase in intraocular pressure. Sensitive persons also may sustain an increase in intraocular pressure with the use of systemic corticosteroid drugs. Diagnostic methods include applanation tonometry (measurement of intraocular pressure), ophthalmoscopic visualization of the optic nerve, and central visual field testing. Measurement of intraocular pressures provides a means of assessing glaucoma risk. Because the condition is usually asymptomatic, persons at risk for open-angle glaucoma should have regular direct ophthalmoscopic examinations, on both eyes, concentrating on the optic disk. Optic disk changes frequently are noted before visual field defects become apparent. The elevation in intraocular pressure in persons with open-angle glaucoma is usually treated pharmacologically or, in cases where pharmacologic treatment fails, by increasing aqueous outflow through a surgically created pathway. Drugs used in the long-term management of glaucoma fall into five classes: β-adrenergic antagonists, prostaglandin analogs, adrenergic agonists, carbonic anhydrase inhibitors, and cholinergic agonists.17 Most glaucoma drugs are applied topically. However, systemic side effects may occur. Topical β-adrenergic antagonists are usually the drugs of first choice for lowering intraocular pressure. The βadrenergic antagonists are thought to lower intraocular pressure by decreasing aqueous humor production in the ciliary body. β-adrenergic antagonists decrease the production of aqueous humor by approximately one third.17 Prostaglandins are locally acting hormones, found in most tissues. At low concentrations, prostaglandin F2α increases 1304 UNIT XIII Special Sensory Function uveoscleral outflow through the iris root and ciliary body, either by decreasing the extracellular matrix or by relaxing the ciliary musculature. A topical prostaglandin analog, latanoprost (Xalatan), is the only drug of this class currently available in the United States.17 Adrenergic agonists cause an early decrease in production of aqueous humor by constricting the vessels supplying the ciliary body. Nonselective adrenergic agents such as epinephrine stimulate both α and β receptors and tend to cause more systemic side effects such as tachycardia than nonselective drugs. Apraclonidine, an α-adrenergic agonist, reduces intraocular pressure by decreasing aqueous humor formation. Dipivefrin is a prodrug converted to epinephrine in the eye and seldom causes side effects. Carbonic anhydrase inhibitors reduce the secretion of aqueous humor by the ciliary epithelium. Until recently, these drugs had to be taken orally, and systemic side effects were common. A topical carbonic anhydrase inhibitor (dorzolamide), which acts locally, is now available. Acetylcholine is the postganglionic neuromediator for the parasympathetic system; it increases aqueous outflow through contraction of the ciliary muscle and pupillary constriction (miosis). Cholinergic drugs exert their effects by increasing the effects of acetylcholine. Acetylcholine is broken down by the enzyme acetylcholinesterase. The most commonly used miotic drug is pilocarpine, which functions as a direct cholinergic agonist. Echothiophate, another miotic agent, acts indirectly by inhibiting the breakdown of acetylcholine by acetylcholinesterase. When a reduction in intraocular pressure cannot be maintained through pharmacologic methods, surgical treatment may become necessary. Until recently, the main surgical treatment for open-angle glaucoma was a filtering procedure in which an opening was created between the anterior chamber and the subconjunctival space. An argon or neodymium–yttrium-aluminum-garnet (Nd:YAG) laser technique, in which multiple spots are applied 360 degrees around the trabecular meshwork, has been developed.12 The microburns resulting from the laser treatment scar rather than penetrate the trabecular meshwork, a process thought to enlarge the outflow channels by increasing the tension exerted on the trabecular meshwork. Cryotherapy, diathermy, and high-frequency ultrasound may be used in some cases to destroy the ciliary epithelium and reduce aqueous humor production. Congenital and Infantile Glaucoma There are several types of childhood glaucoma including congenital glaucoma that is present at birth and infantile glaucoma that develops during the first 2 to 3 years of life. As with glaucoma in adults, childhood glaucoma can occur as a primary or secondary disorder. Congenital glaucoma is caused by a disorder in which the anterior chamber retains its fetal configuration, with aberrant trabecular meshwork extending to the root of the iris, or is covered by a membrane. In general, it has a much poorer prognosis than infantile glaucoma. Primary infantile glaucoma occurs in approximately 1 in 10,000 live births but accounts for 2% to 15% of persons in institutions for the blind.18 It is bilateral in 65% to 80% of cases and occurs more commonly in males than females. About 10% of cases have a familial origin, and the rest are either sporadic or possibly multifactorial with reduced penetrance. The familial cases are usually transmitted as an autosomal dominant trait with potentially high penetrance. Recent studies suggest a mutation in chromosome 2 (2p21 region). This gene is expressed in the tissues of the anterior chamber of the eye and its protein product plays an important role in the metabolism of molecules that are used in signaling pathways during the terminal stages of anterior chamber development.18 The earliest symptoms of congenital or infantile glaucoma are excessive lacrimation and photophobia. Affected infants tend to be fussy, have poor eating habits, and rub their eyes frequently. Diffuse edema of the cornea usually occurs, giving the eye a grayish-white appearance. Chronic elevation of the intraocular pressure before the age of 3 years causes enlargement of the entire globe (i.e., buphthalmos). Early surgical treatment is necessary to prevent blindness. In summary, glaucoma is a leading cause of blindness in the United States. It is characterized by conditions that cause an increase in intraocular pressure and that, if untreated, can lead to atrophy of the optic disk and progressive blindness. The aqueous humor is formed by the ciliary epithelium in the posterior chamber and flows through the pupil to the angle formed by the cornea and the iris. Here, it filters through the trabecular meshwork and enters the canal of Schlemm for return to the venous circulation. Glaucoma results from overproduction or the impeded outflow of aqueous humor from the anterior chamber of the eye. Two major forms of glaucoma exist: angle closure and open angle. Angle-closure glaucoma is caused by a narrow anterior chamber and blockage of the outflow channels at the angle formed by the iris and the cornea. This occurs when the iris becomes thickened during pupillary dilation. Open-angle glaucoma is caused by microscopic obstruction of the trabecular meshwork. Open-angle glaucoma is usually asymptomatic, and considerable loss of the visual field often occurs before medical treatment is sought. Routine screening by applanation tonometry provides one of the best means for early detection of glaucoma before vision loss has occurred. Congenital glaucoma is caused by a disorder in which the anterior chamber retains its fetal configuration, with aberrant trabecular meshwork extending to the root of the iris, or is covered by a membrane. It occurs in approximately 1 in 10,000 live births but accounts for 2% to 15% of persons in institutions for the blind. Disorders of the Lens and Lens Function After completing this section of the chapter, you should be able to meet the following objectives: ✦ Describe the changes in eye structure that occur with cataract CHAPTER 54 Disorders of Visual Function 1305 ✦ Cite risk factors associated with cataract ✦ Characterize the visual changes that occur with cataract ✦ Describe the treatment of persons with cataracts The function of the eye is to transform light energy into nerve signals that can be transmitted to the cerebral cortex for interpretation. Optically, the eye is similar to a camera. It contains a lens system that inverts an image, an aperture (i.e., the pupil) for controlling light exposure, and a retina that corresponds to the film and records the image. A DISORDERS OF REFRACTION AND ACCOMMODATION The lens is an avascular, transparent, biconvex body, the posterior side of which is more convex than the anterior side. A thin, highly elastic lens capsule is attached to the surrounding ciliary body by delicate suspensory radial ligaments called zonules, which hold the lens in place (see Fig. 54-5). In providing for a change in lens shape, the tough elastic sclera acts as a bow, and the zonule and the lens capsule act as the bowstring. The suspensory ligaments and lens capsule are normally under tension, causing the lens to have a flattened shape for distant vision. Contraction of the muscle fibers of the ciliary body narrows the diameter of the ciliary body, relaxes the fibers of the suspensory ligaments, and allows the lens to relax to a more spherical or convex shape for near vision. When light passes from one medium to another, its velocity is decreased or increased, and the direction of light transmission is changed. This change in direction of light rays is called refraction. When light rays pass through the center of a lens, their direction is not changed; however, other rays passing peripherally through a lens are bent (Fig. 54-8). The refractive power of a lens is usually described as the distance (in meters) from its surface to the point at which the rays come into focus (i.e., focal length). Usually, this is reported as the reciprocal of this distance (i.e., diopters).13 For example, a lens that brings an object into focus at 0.5 m has a refractive power of 2 diopters (1.0/0.5 = 2.0). With a fixed-power lens, the closer an object is to the lens, the further behind the lens is its focus point. The closer the object, the stronger and more precise the focusing system must be. In the eye, the major refraction of light begins at the convex corneal surface. Further refraction occurs as light moves from the posterior corneal surface to the aqueous humor, from the aqueous humor to the anterior lens surface, from the anterior lens surface to the posterior lens surface, and from the posterior lens surface to the vitreous humor. Disorders of Refraction A perfectly shaped optic globe and cornea result in optimal visual acuity, producing a sharp image in focus at all points on the retinal surface in the posterior part, or fundus, of the eye (see Fig. 54-8). Unfortunately, individual differences in formation and growth of the eyeball and cornea frequently result in inappropriate focal image formation. If the anteroposterior dimension of the eyeball is B C FIGURE 54-8 (A) Accommodation. The solid lines represent rays of light from a distant object, and the dotted lines represent rays from a near object. The lens needs to be flatter for the former and more convex for the latter. In each case, the rays of light are brought to a focus on the retina. (B) Hyperopia corrected by a biconvex lens, shown by the dotted lines. (C) Myopia corrected by a biconcave lens, shown too short, the image is focused posterior to (behind) the retina. This is called hyperopia or farsightedness. In such cases, the accommodative changes of the lens can bring distant images into focus, but near images become blurred. Hyperopia is corrected by appropriate biconvex lenses. If the anteroposterior dimension of the eyeball is too long, the focus point for an infinitely distant target is anterior to the retina. This condition is called myopia or nearsightedness (see Fig. 54-8). Persons with myopia can see close objects without problems because accommodative changes in their lens bring near objects into focus, but distant objects are blurred. Myopia can be corrected with an appropriate biconcave lens. Radial keratotomy, a form of refractive corneal surgery, can be performed to correct the defect. This surgical procedure involves the use of radial incisions to alter the corneal curvature. Refractive defects of the corneal surface do not permit the formation of a sharp image. Nonuniform curvature of 1306 UNIT XIII Special Sensory Function the refractive medium (e.g., horizontal vs. vertical plane) is called astigmatism. Astigmatism is usually the result of a defect in the cornea, but it can result from defects in the lens or the retina. Spherical aberration, another refractive error, involves a cornea with nonspherical surfaces. Lens correction is available for both refractive errors. Disorders of Accommodation Because the retina is at a fixed distance from the lens, adjustability in the refractive power of the lens is needed so that a clear image is maintained as gaze is shifted from afar to a near object. The process by which the refractory power of the lens is increased and the diverging light rays are bent more sharply is called accommodation. Accommodation requires convergence of the eyes, pupillary constriction, and thickening of the lens through contraction of the ciliary muscle. Contraction of the ciliary muscles is controlled mainly by the parasympathetic fibers of the oculomotor cranial nerve (CN III). In near vision, pupillary constriction (i.e., miosis) improves the clarity of the retinal image. This must be balanced against the resultant decrease in light intensity reaching the retina. During changes from near to far vision, pupillary dilation partially compensates for the reduced size of the retinal image by increasing the light entering the pupil. A third component of accommodation involves the reflex narrowing of the palpebral opening during near vision and widening during far vision. Paralysis of the ciliary muscle, with loss of accommodation, is called cycloplegia. Pharmacologic cycloplegia is sometimes necessary to aid ophthalmoscopic examination of the fundus of the eye, especially in small children who are unable to hold a steady fixation during the examination. Lens shape is totally controlled by the pretectal region and the parasympathetic pathways through the oculomotor nerve to the ciliary muscle. Accommodation is lost with destruction of this pathway. The term presbyopia refers to changes in vision that occur because of aging. The lens consists of transparent fibers arranged in concentric layers, of which the external layers are the newest and softest. No loss of lens fibers occurs with aging; instead, additional fibers are added to the outermost portion of the lens. As the lens ages, it thickens, and its fibers become less elastic, so that the range of focus or accommodation is diminished to the point at which reading glasses become necessary for near vision. CATARACTS A cataract is a lens opacity that interferes with the transmission of light to the retina. It has been estimated that 13 million persons in the United States 40 years of age or older are visually disabled because of cataracts.1 Cataracts are the most common cause of age-related visual loss in the world; they are found in approximately 50% of those between 65 and 74 years of age and in 70% of those older than 75 years.1 Cataract surgery is the most common surgical procedure covered by Medicare, with more than 1 million procedures performed annually. More than 90% of persons undergoing cataract surgery experience visual improvement if there is no ocular comorbidity.19 Causes and Types of Cataracts The cause of cataract development is thought to be multifactorial, with different factors being associated with different types of opacities. The pathogenesis of cataracts is not completely understood. Several risk factors have been proposed, including the effects of aging, genetic influences, environmental and metabolic influences, drugs, and injury.20 Metabolically induced cataracts are caused by disorders of carbohydrate metabolism (diabetes) or inborn errors of metabolism.21 In a condition called Lowe’s syndrome, abnormal synthesis of lens proteins leads to opacification. Long-term exposure to sunlight (ultraviolet B radiation) and heavy smoking has been associated with increased risk for cataract formation.20 Occasionally, cataracts occur as a developmental defect (i.e., congenital cataracts) or secondary to trauma or diseases. Cataracts can result from several drugs. Corticosteroid drugs have been implicated as causative agents in cataract formation. Both systemic and inhaled corticosteroids have been cited as risk factors.22,23 Other drugs associated with cataracts include the phenothiazines, amiodarone, and strong miotic ophthalmic drugs such as phospholine iodine.20 Frequent examination of lens transparency should accompany the use of these and any other medications with potential cataract-forming effects. Traumatic Cataract. Traumatic cataracts are most often caused by foreign body injury to the lens or blunt trauma to the eye. Foreign body injury that interrupts the lens capsule allows aqueous and vitreous humor to enter the lens and initiate cataract formation. Other causes of traumatic cataract are overexposure to heat (e.g., glassblower’s cataract) or to ionizing radiation. The radiation dose necessary to cause a cataract varies with the amount and type of energy; younger lenses are most vulnerable. Congenital Cataract. A congenital cataract is one that is present at birth. Among the causes of congenital cataracts are genetic defects, toxic environmental agents, and viruses such as rubella.8 A maternal rubella infection during the first trimester of pregnancy can cause congenital cataract. Cataracts and other developmental defects of the ocular apparatus depend on the total dose and the embryonic stage at the time of exposure. During the last trimester of pregnancy, genetically or environmentally influenced malformation of the superficial lens fibers can occur. Congenital lens opacities may occur in children of diabetic mothers. Most congenital cataracts are not progressive and are not dense enough to cause significant visual impairment. However, if the cataracts are bilateral and the opacity is significant, lens extraction should be done on one eye by the age of 2 months to permit the development of vision and prevent nystagmus. If the surgery is successful, the contralateral lens should be removed soon after. Senile Cataract. Cataract is the most common cause of age-related vision loss in the world.24 With normal aging, the nucleus and the cortex of the lens enlarge CHAPTER 54 as new fibers are formed in the cortical zones of the lens. In the nucleus, the old fibers become more compressed and dehydrated. Metabolic changes also occur. Lens proteins become more insoluble, and concentrations of calcium, sodium, potassium, and phosphate increase. During the early stages of cataract formation, a yellow pigment and vacuoles accumulate in the lens fibers. The unfolding of protein molecules, cross-linking of sulfhydryl groups, and conversion of soluble to insoluble proteins lead to the loss of lens transparency. The onset is usually gradual, and the only symptoms are increasingly blurred vision and visual distortion. Manifestations The manifestations of cataract depend on the extent of opacity and whether the defect is bilateral or unilateral. With the exception of traumatic or congenital cataract, most cataracts are bilateral. Age-related cataracts, which are the most common type, are characterized by increasingly blurred vision and visual distortion. Vision for far and near objects decreases. Dilation of the pupil in dim light improves vision. With nuclear cataracts (those involving the lens nucleus), the refractive power of the anterior segment often increases to produce an acquired myopia. Persons with hyperopia may experience a “second sight” or improved reading acuity until increasing opacity reduces acuity. Central lens opacities may divide the visual axis and cause an optical defect in which two or more blurred images are seen. Posterior subcapsular cataracts are located in the posterior cortical layer and usually involve the central visual axis. In addition to decreased visual acuity, cataracts tend to cause light entering the eye to be scattered, thereby producing glare or the abnormal presence of light in the visual field. Diagnosis and Treatment Diagnosis of cataract is based on ophthalmoscopic examination and the degree of visual impairment on the Snellen vision test. On ophthalmoscopic examination, cataracts may appear as a gross opacity filling the pupillary aperture or as an opacity silhouetted against the red background of the fundus. A Snellen test acuity of 20/50 is a common requirement for drivers of motor vehicles. Other tests of potential vision (e.g., the ability to see well after surgery), such as electrophysiologic testing in which the response to visual stimuli is measured electronically, may be done. There is no effective medical treatment for cataract. Use of strong bifocals, magnification, appropriate lighting, and visual aids may be used as the cataract progresses. Surgery is the only treatment for correcting cataract-related vision loss. Surgery usually involves lens extraction and intraocular lens implantation. It is commonly performed on an outpatient basis with the use of local anesthesia. The use of extracapsular surgery, which leaves the posterior capsule of the lens intact, has significantly improved the outcomes of cataract surgery. The cataract lens is usually removed using phacoemulsification techniques.20,23 Phacoemulsification involves ultrasonic fragmentation of the lens into fine pieces, which then are aspirated from the eye. Disorders of Visual Function 1307 One of the greatest advances in cataract surgery has been the development of reliable intraocular implants. Until recently, only monofocal intraocular lenses that correct for distance vision were available, and eyeglasses were needed for near vision. This has been remedied by the introduction of multifocal lenses. In summary, the lens is a biconvex, avascular, colorless, and almost transparent structure suspended behind the iris. The shape of the lens is controlled by the ciliary muscle, which contracts and relaxes the zonule fibers, thus changing the tension on the lens capsule and altering the focus of the lens. Refraction, which refers to the ability to focus an object on the retina, depends on the size and shape of the eyeball and the cornea and on the focusing ability of the lens. Errors in refraction occur when the visual image is not focused on the retina because of individual differences in the size or shape of the eyeball or cornea. In hyperopia, or farsightedness, the image falls behind the retina. In myopia, or nearsightedness, the image falls in front of the retina. Accommodation is the process by which a clear image is maintained as the gaze is shifted from afar to a near object. It requires convergence of the eyes, pupillary constriction, and thickening of the lens through contraction of the ciliary muscle. Presbyopia is a change in the lens that occurs because of aging such that the lens becomes thicker and less able to change shape and accommodate for near vision. A cataract is a lens opacity. It can occur as the result of congenital influences, metabolic disturbances, infection, injury, and aging. The most common type of cataract is the senile cataract that occurs with aging. Treatment of a totally opaque or mature cataract is surgical extraction. An intraocular lens implant may be inserted during the surgical procedure to replace the removed lens; otherwise, thick convex lenses or contact lenses are used to compensate for the loss of lens function. Disorders of the Vitreous and Retina After completing this section of the chapter, you should be able to meet the following objectives: ✦ Relate the phagocytic function of the retinal pigment epithelium to the development of retinitis pigmentosa ✦ Cite the manifestations and long-term visual effects of papilledema and central artery and central venous occlusions ✦ Describe the pathogenesis of background and proliferative diabetic retinopathies and their mechanisms of visual impairment ✦ Discuss the cause of retinal detachment ✦ Explain the pathology and visual changes associated with macular degeneration The posterior segment, comprising five sixths of the eyeball, contains the transparent vitreous humor and the neural retina. The posterior aspect of the retina, the fundus, is visualized through the pupil with an ophthalmoscope. 1308 UNIT XIII Special Sensory Function DISORDERS OF THE VITREOUS Vitreous humor (i.e., vitreous body) is a colorless, amorphous biologic gel that fills the posterior cavity of the eye. It consists of approximately 99% water, some salts, glycoproteins, proteoglycans, and dispersed collagen fibrils. The vitreous is attached to the ciliary body and the peripheral retina in the region of the ora serrata and to the periphery of the optic disk. Disease, aging, and injury can disturb the factors that maintain the water of the vitreous humor in suspension, causing liquefaction of the gel to occur. With the loss of gel structure, fine fibers, membranes, and cellular debris develop. When this occurs, floaters (images) can often be noticed as these substances move within the vitreous cavity during head movement. Blood vessels may grow from the surface of the retina or optic nerve into the posterior surface of the vitreous, and cause bleeding into the vitreous cavity. In a procedure called a vitrectomy, the removal and replacement of the vitreous with a balanced saline solution can restore sight in some persons with vitreous opacities resulting from hemorrhage or vitreoretinal membrane formations that cause legal blindness. Using this procedure, a small probe with a cutting tip is used to remove the opaque vitreous and membranes. The procedure is difficult and requires complex instrumentation. It is of no value if the retina is not functional. DISORDERS OF THE RETINA The function of the retina is to receive visual images, partially analyze them, and transmit this modified information to the brain.11 It is composed of two layers: the outer, melanin-containing layer and the inner neural layer. The Direction of light light-sensitive neural retina covers the inner aspect of the eyeball. A non–light-sensitive portion of the retina, along with the retinal pigment epithelium, continues anteriorly to form the posterior surface of the iris. A wavy border called the ora serrata exists at the junction between the light-sensitive and the non–light-sensitive retinas. Separating the vascular portion of the choroid from the single layer of pigmented cells is a thin layer of elastic tissue, Bruch’s membrane, which contains collagen fibrils in its superficial and deep portions. Cells of the pigmented layer receive their nourishment by diffusion from the choroid vessels. Tight junctions between the endothelial cells of the retinal blood vessels combine to form a blood–retina barrier. Disorders of the retina and its function include derangements of the pigment epithelium (e.g., retinitis pigmentosa); ischemic conditions caused by disorders of the retinal blood supply; disorders of the retinal vessels such as retinopathies that cause hemorrhage and the development of opacities; separation of the pigment and sensory layers of the retina (i.e., retinal detachment); retinopathy of prematurity; and abnormalities of Bruch’s membrane and choroid (e.g., macular degeneration). Because the retina has no pain fibers, most diseases of the retina are painless. The Neural Retina The neural retina is composed of three layers of neurons: a posterior layer of photoreceptors, a middle layer of bipolar cells, and an inner layer of ganglion cells that communicate with the photoreceptors (Fig. 54-9). A pattern of light on the retina falls on a massive array of photoreceptors. These photoreceptors synapse with bipolar and other interneurons before action potentials in ganglion cells relay the message to specific regions of the brain and the brain stem associated with vision. For rods, this micro- Axons of retinal ganglion cells Optic disk Ganglion cell layer Bipolar cell layer Sclera Optic nerve Layer of rods and cones Pigment epithelium FIGURE 54-9 Organization of the human retina. The visual pathway begins with photoreceptors (rods and cones) in the retina. The responses of the photoreceptors are transmitted by the bipolar cells to the ganglion cell layer of the retina. CHAPTER 54 circuitry involves the convergence of signals from many rods on a single ganglion cell. This arrangement maximizes spatial summation and the detection of stimulated (light vs. dark) receptors. The interneurons, composed of horizontal and amacrine cells, have cell bodies in the bipolar layer, and they play an important role in modulating retinal function. A superficial marginal layer contains the axons of the ganglion cells as they collect and leave the eye by way of the optic nerve (see Fig. 54-9). These fibers lie beside the vitreous humor. Light must pass through the transparent inner layers of the sensory retina before it reaches the photoreceptors. Photoreceptors Two types of photoreceptors are present in the retina: rods, capable of black–white discrimination, and cones, capable of color discrimination.11 Both types of photoreceptors are thin, elongated, mitochondria-filled cells with a single, highly modified cilium (Fig. 54-10). The cilium has a short base, or inner segment, and a highly modified outer segment. The plasma membrane of the outer segment is tightly folded to form membranous disks (rods) or conical shapes (cones) containing visual pigment. These disks are continuously synthesized at the base of the outer segment and shed at the distal end. Discarded membranes Synaptic body Nucleus Disorders of Visual Function 1309 are phagocytized by the retinal pigment cells. If this phagocytosis is disrupted, as in retinitis pigmentosa, the sensory retina degenerates. Rods. Photoreception involves the transduction of light energy into an altered ionic membrane potential of the rod cell. Light passing through the eye penetrates the nearly transparent neural elements to produce decomposition of the photochemical substance (visual pigment) called rhodopsin in the outer segment of the rod. Light that is not trapped by a rhodopsin molecule is absorbed by the retinal pigment melanin or the more superficial choroid melanin. Rhodopsin consists of a protein called opsin and a vitamin A–derived pigment called retinal. During light stimulation, rhodopsin is broken down into its component parts, opsin and retinal; retinal subsequently is converted into vitamin A. The reconstitution of rhodopsin occurs during total darkness; vitamin A is transformed into retinal, and then opsin and retinal combine to form rhodopsin. Considerable stores of vitamin A are present in the retinal pigment cells and in the liver; therefore, a vitamin A deficiency must be present for weeks or months to affect the photoreceptive process. Reduced sensitivity to light, a symptom of vitamin A deficiency, initially affects night vision; however, this is quickly reversed by injection or ingestion of the vitamin. Rod-based vision is particularly sensitive to detecting light, especially moving light stimuli, at the expense of clear pattern discrimination. Rod vision is particularly adapted for night and low-level illumination. Dark adaptation is the process by which rod sensitivity increases to the optimum level. This requires approximately 4 hours in total or near-total darkness and involves only rods or scotopic vision (night vision). During daylight or high-intensity bombardment, the concentration of vitamin A increases, and the concentration of the photopigment retinal decreases. During dark adaptation, increased synthesis of retinal from vitamin A results in a higher concentration of rhodopsin for capture of light energy. Cones and Color Sensitivity. Cone receptors that are selec- Inner segment Connecting structure Outer segment FIGURE 54-10 Retinal rod, showing its component parts and the distribution of its organelles. Its outer segment contains the disks (rods). The connecting structure joins the outer and inner segments. The inner segment contains the mitochondria, the ribosomal endoplasmic reticulum, the free ribosomes, and the Golgi saccules. The synaptic body forms the site where the photo receptors synapse with other nerve cells. tively sensitive to different wavelengths of light provide the basis for color vision. Three types of cones, or conecolor systems, respond to the blue, green, and red portions of the visible electromagnetic spectrum. This selectivity reflects the presence of one of three color-sensitive molecules to which the photochemical substance (visual pigment) is bound. The decomposition and reconstitution processes of the cone visual pigments are believed to be similar to that of the rods. The color a person perceives depends on which set of cones or combination of sets of cones is stimulated in a given image. Cones do not have the dark adaptation of rods. Consequently, the dark-adapted eye is a rod receptor eye with only black-gray-white experience (scotopic or night vision). The light-adapted eye ( photopic vision) adds the capacity for color discrimination. Rhodopsin has its maximum sensitivity in the blue-green region of the electromagnetic spectrum. If red lenses are worn in daylight, the red cones (and green cones to some extent) are in use, whereas the 1310 UNIT XIII Special Sensory Function rods and blue cones are essentially in the dark, and therefore dark adaptation proceeds. This method is used by military and night-duty airport control tower personnel to allow adaptation to take place before they go on duty in the dark. Macula and Fovea. An area approximately 1.5 mm in diameter near the center of the retina, called the macula lutea (i.e., “yellow spot”), is especially adapted for acute and detailed vision.11 This area is composed entirely of cones. In the central portion of the macula, the fovea centralis (foveola), blood vessels, and innermost layers are displaced to one side instead of resting on top of the cones (Fig. 54-11). This allows light to pass unimpeded to the cones without passing through several layers of the retina. Of the approximately 6.4 million cones found in the retina, 200,000 cones are located in the fovea. The density of cones drops off rapidly away from the fovea. Rods are not present in the fovea, but their numbers increase as the cones decrease in density toward the periphery of the retina. An estimated 110 to 125 million rods are found in the retina. Many cones are connected one-to-one with ganglion cells. Retinal microcircuitry for cones emphasizes the detection of edges. This type of circuitry favors high acuity. A concentration of acuity-favoring cones at the fovea supports the use of this part of the retina for fine analysis of focused central vision. It is not until the age of 4 years that the fovea is fully developed. Color Blindness. Color blindness is a misnomer for a condition in which persons appear to confuse or mismatch colors or experience reduced acuity for color discrimination. Such persons are often unaware of their defect until they attempt to discriminate between red and green traf- fic lights or show difficulty matching colors. Most often the result of genetic factors, the deficit can result from the defective function of one or more of the three color-cone mechanisms. The deficiency is usually partial but can be complete. Rarely are two of the color mechanisms missing; when this occurs, usually red and green are missing. Persons with no color mechanisms are rare. For them, the world is experienced entirely as black, gray, and white. The genetically color-blind person has never experienced the full range of normal color vision and is unaware of what he or she is missing. Color discrimination is necessary for everyday living, and color-blind persons, knowingly or unknowingly, make color discriminations based on other criteria, such as brightness or position. For example, the red light of a traffic signal is always the upper light, and the green is the lower light. Color-blind persons experience difficulties when brightness differences are small and discrimination must be based on hue and saturation qualities. The genes responsible for color blindness affect receptor mechanisms rather than central acuity. The gene for the red and green mechanisms is sex linked (i.e., on the X chromosomes), resulting in a much higher incidence among males of red, green, or red-green color blindness; however, the gene affecting the blue mechanism is autosomal. Acquired color defects are more complex but follow a general rule: disease of the more peripheral retina affects blue discrimination, and disease of the more central retina affects red and green discrimination because blue cones are not present in the central fovea. Retinitis Pigmentosa. Retinitis pigmentosa (RP) is a group of hereditary diseases that cause slow degenerative changes in the retinal photoreceptors.25,26 There are several modes FIGURE 54-11 Location of fovea in the retina. (Kandel E.R., Schwartz J.H., Jessell T.M. [1991]. Principles of neural science [3rd ed.]. New York: Elsevier) CHAPTER 54 of inheritance, including autosomal dominant, autosomal recessive, sex linked, and sporadic. With the advances of gene technology, there are now 36 known or predicted RP genes.25 The most common group of these mutated genes are those encoding proteins in the visual cascade of the photoreceptor outer segment. In typical cases, known as rod-cone RP, the rods are the predominantly affected photoreceptor cells. This generally produces a number of characteristic clinical symptoms including night blindness, which is usually an early symptom, and bilateral symmetric loss of mid-peripheral fields. Although there is relative preservation of macular vision, the visual field defects gradually increase both centrally and peripherally. With progression, cone photoreceptor cells are also affected, and day vision and central visual acuity are compromised. The rate of visual failure is variable. Some people are severely visually handicapped before the age of 20, whereas others experience few symptomatic visual deficits even after age 70.26 Disorders of Retinal Blood Supply The blood supply for the retina is derived from two sources: the choriocapillaris of the choroid and the branches of the central retinal artery (Fig. 54-12). Oxygen and other nutritional substances needed by the retina and its component parts (pigment cells, rods and cones) are supplied by diffusion from blood vessels in the choroid. Because the choriocapillary layer provides the only blood supply for the fovea centralis (i.e., foveola), detachment of this part of the sensory retina from the pigment epithelium causes irreparable visual loss. The bipolar, horizontal, amacrine, and ganglion cells, and the ganglion cell axons that gather at the optic disk, are supplied by branches of the central retinal artery.27 The 1311 Disorders of Visual Function DISORDERS OF THE RETINAL BLOOD SUPPLY ➤ The blood supply for the retina is derived from the central retinal artery, which supplies blood flow for the entire inside of the retina, and from vessels in the choroid, which supply the rods and cones. ➤ Central retinal occlusion interrupts blood flow to the inner retina and results in unilateral blindness. ➤ The retinopathies, which are disorders of the retinal vessels, interrupt blood flow to the visual receptors, leading to visual impairment. ➤ Retinal detachment separates the visual receptors from the choroid, which provides their major blood supply. central artery of the retina is a branch of the ophthalmic artery. It enters the globe through the optic disk. Branches of this artery radiate over the entire retina, except the central fovea, which is surrounded by, but is not crossed by, arterial branches. The central retinal artery is an end artery meaning that it does not anastomose with other arteries. This is critical because an infarct in this artery will totally deprive distal structures of their vascular supply. Retinal veins follow a distribution parallel to the arterial branches and carry venous blood to the central vein of the retina, which exits the back of the eye through the optic disk. Funduscopic examination of the eye with an ophthalmoscope provides an opportunity to examine the retinal blood vessels and other aspects of the retina (Fig. 54-13). Because the retina is an embryonic outgrowth of the brain Vitreous chamber (contains vitreous humor) Sclera Choroid Retina Fovea centralis Macula lutea Central retinal vein Central retinal artery Optic nerve Posterior ciliary artery Optic disk (blind spot) Retinal blood vessels FIGURE 54-12 Retinal circulation. 1312 UNIT XIII Special Sensory Function FIGURE 54-13 Fundus of the eye as seen in retinal examination with an ophthalmoscope: (left) normal fundus; (middle) diabetic retinopathy—combination of microaneurysms, deep hemorrhages, and hard exudates of background retinopathy; (right) hypertensive retinopathy with purulent exudates. Some exudates are scattered, while others radiate from the fovea to form a macular star. (Bates B. [1995]. A guide to physical examination and history taking. (pp. 208, 210). Philadelphia: J.B. Lippincott) and the blood vessels are to a considerable extent representative of brain blood vessels, the ophthalmoscopic examination of the fundus of the eye permits the study and diagnosis of metabolic and vascular diseases of the brain and of pathologic processes that are specific to the retina. Functioning of the retina, like that of other cellular portions of the central nervous system (CNS), depends on an oxygen supply from the vascular system. One of the earliest signs of decreased perfusion pressure in the head region is a graying-out or blackout of vision, which usually precedes loss of consciousness. This can occur during large increases in intrathoracic pressure, which interfere with the return of venous blood to the heart, as occurs with Valsalva’s maneuver; with systemic hypotension; and during sudden postural movements (e.g., postural hypotension). Ischemia of the retina occurs during general circulatory collapse. If a person survives cardiopulmonary arrest, for instance, permanently decreased visual acuity can occur as a result of edema and the ischemic death of retinal neurons. This is followed by primary optic nerve atrophy proportional to the extent of ganglionic cell death. The ophthalmic artery, the source of the central retinal artery, takes its origin from the internal carotid artery.27 Intermittent retinal ischemia can accompany internal carotid or common carotid stenosis. Amaurosis fugax is characterized by transient episodes of monocular visual loss lasting 5 to 10 minutes.27 Persons with the disorder often describe a curtain coming down from above or across their vision, usually with complete return of vision within seconds or minutes. Besides the vision loss, contralateral hemiplegia or sensory deficits may accompany the episodes. The condition, commonly due to emboli, is most often the result of carotid artery disease.27 Papilledema. The central retinal artery enters the eye through the optic papilla in the center of the optic nerve. An accompanying vein exits the eye along the same path. The entrance and exit of the central artery and vein of the retina through the tough scleral tissue at the optic papilla can be compromised by any condition causing persistent increased intracranial pressure. The most common of these conditions are cerebral tumors, subdural hematomas, hydrocephalus, and malignant hypertension. Usually, the thin-walled, low-pressure veins are the first to collapse, with the consequent backup and slowing of arterial blood flow. Under these conditions, capillary permeability increases, and leakage of fluid results in edema of the optic papilla, called papilledema. The interior surface of the papilla is normally cup shaped and can be evaluated through an ophthalmoscope. With papilledema, sometimes called choked disk, the optic cup is distorted by protrusion into the interior of the eye (Fig. 54-14). Because this sign does not occur until the intracranial pressure is significantly elevated, compression damage to the optic nerve fibers passing through the lamina cribrosa may have FIGURE 54-14 Chronic papilledema. The optic nerve head is congested and protrudes anteriorly toward the interior of the eye. It has blurred margins, and vessels within it are poorly seen. (Rubin E., Farber J.L. [1999]. Pathology [3rd ed., p. 1558]. Philadelphia: Lippincott-Raven) CHAPTER 54 Disorders of Visual Function 1313 begun. As a warning sign, papilledema occurs quite late. Unresolved papilledema results in the destruction of the optic nerve axons and blindness. Microhemorrhages in the layer of ganglionic cell axon bundles result in the appearance of cotton-wool spots on the fundus. Central Retinal Artery Occlusion. Complete occlusion of Retinopathies the central artery of the retina results in sudden unilateral blindness. This is an uncommon disorder of older persons and most often is caused by embolism or atherosclerosis. Because the retina has a dual blood supply, the survival of retinal structures is possible if blood flow can be reestablished within approximately 90 minutes.27 If blood flow is not restored, the infarcted retina swells and opacifies. Because the receptors of the central fovea are supplied with blood from the choroid, they survive (i.e., macular sparing). A cherry-red spot, indicating a healthy fovea, is surrounded by the pale white, opacified retina.31 Although the nerve fibers of the optic disk are adequately supplied by the choroid, the disk becomes pale after death of the ganglion cells and their axonal processes (i.e., optic nerve fibers), resulting in optic nerve atrophy. Occlusions of branches of the central artery, called branch arterial occlusions, are essentially retinal strokes. These occur mainly from emboli and local infarction in the neural retina. The opacification that follows is often slowly resolved, and retinal transparency is restored. Local blind spots or scotomas may occur after destruction of local elements of the retina. Loss of the axons of destroyed ganglion cells results in some optic nerve atrophy. Central Retinal Vein Occlusion. Occlusion of the central retinal vein results in venous dilation, stasis, and reduced flow through the retinal veins. It is usually monocular and causes painless rapid deterioration of visual acuity because of an accompanying increase in capillary wall fragility and a lack of arterial inflow.27 Superficial and deep hemorrhages may occur throughout the retina. Among the causes of central retinal vein obstruction are hypertension, diabetes mellitus, and conditions such as sickle cell anemia that slow venous blood flow. The reduction in blood flow results in neovascularization with fibrovascular invasion of the space between the retina and the vitreous humor. Besides obstructing normal visual function, the new vessels are fragile and prone to hemorrhage. Escaped blood may fill the space between the retina and vitreous, producing the appearance of a sudden veil over the visual field. The blood can find its way into the aqueous humor (i.e., hemorrhagic glaucoma). Photocoagulation of the spreading new blood vessels with high-intensity light or laser beam is used to prevent blindness and eye pain. As the hemorrhage is resolved, degenerating blood products can produce contraction of the vitreous and formation of fibrous tissue within it, causing tears and detachment of the retina. Much more common are local vein occlusions with regional and focal capillary microhemorrhages that produce the same but more restricted pathologic effects. These microhemorrhages result in the formation of rings of yellow exudate composed of lipid and lipoprotein blood breakdown products. Microhemorrhages deep in the neural retina are somewhat restricted by the vertical organization of the neural elements and result in dot hemorrhages. Disorders of the retinal vessels result in microaneurysms, neovascularization, hemorrhage, and formation of retinal opacities. Microaneurysms are outpouchings of the retinal vasculature. On ophthalmoscopic examination, they appear as minute, unchanging red dots associated with blood vessels. These microaneurysms tend to leak plasma, resulting in localized edema that gives the retina a hazy appearance. Microaneurysms can be identified with certainty using fluorescein angiography; the fluorescein dye is injected intravenously, and the retinal vessels subsequently are photographed using a special ophthalmoscope and fundus camera. The microaneurysms may bleed, but areas of hemorrhage and edema tend to clear spontaneously. However, they reduce visual acuity if they encroach on the macula and cause degeneration before they are absorbed. Neovascularization involves the formation of new blood vessels. They can develop from the choriocapillaris, extending between the pigment layer and the sensory layer, or from the retinal veins, extending between the sensory retina and the vitreous cavity and sometimes into the vitreous. These new blood vessels are fragile, leak protein, and are likely to bleed. Neovascularization occurs in many conditions that impair retinal circulation, including stasis because of hyperviscosity of blood or decreased flow, vascular occlusion, sickle cell disease, sarcoidosis, diabetes mellitus, and retinopathy of prematurity. Although the cause of neovascularization is uncertain, research links the process with a vascular endothelial growth factor (VEGF) produced by the lining of blood vessels.28,29 The vitreous humor is thought to contain a substance that normally inhibits neovascularization, and this factor apparently is suppressed under conditions in which the new blood vessels invade the vitreous cavity. It is likely that other growth factors and signaling systems are also involved. Hemorrhage can be preretinal, intraretinal, or subretinal. Preretinal hemorrhages occur between the retina and the vitreous. These hemorrhages are usually large because the blood vessels are only loosely restricted; they may be associated with a subarachnoid or subdural hemorrhage and are usually regarded as a serious manifestation of the disorder. They usually reabsorb without complications unless they penetrate into the vitreous. Intraretinal hemorrhages occur because of abnormalities of the retinal vessels, diseases of the blood, increased pressure in the retinal vessels, or vitreous traction on the vessels. Systemic causes include diabetes mellitus, hypertension, and blood dyscrasias. Subretinal hemorrhages are those that develop between the choroid and pigment layer of the retina. A common cause of subretinal hemorrhage is neovascularization. Photocoagulation may be used to treat microaneurysms and neovascularization. Light normally passes through the transparent inner portions of the sensory retina before reaching the photoreceptors. Opacities such as hemorrhages, exudate, cottonwool patches, and edema can produce a localized loss of 1314 UNIT XIII Special Sensory Function transparency observable with an ophthalmoscope. Exudates are opacities resulting from inflammatory processes. The development of exudates often results in the destruction of the underlying retinal pigment and choroid layer. Deposits are localized opacities consisting of lipidladen macrophages or accumulated cellular debris. Cotton-wool patches are retinal opacities with hazy, irregular outlines. They occur in the nerve fiber layer and contain cell organelles. Cotton-wool patches are associated with retinal trauma, severe anemia, papilledema, and diabetic retinopathy. Diabetic Retinopathy. Diabetic retinopathy is the third leading cause of blindness for all ages in the United States. It ranks first as the cause of newly reported cases of blindness in persons between the ages of 20 and 74 years. During the first two decades of the disease, nearly all persons with type 1 diabetes and more than 60% of persons with type 2 diabetes have retinopathy.30 Diabetic retinopathy can be divided into two types: nonproliferative (i.e., background) and proliferative.31 Back- ground or nonproliferative retinopathy is confined to the retina. It involves thickening of the retinal capillary walls and microaneurysm formation (Fig. 54-15). Ruptured capillaries cause small intraretinal hemorrhages, and microinfarcts may cause cotton-wool exudates. A sensation of glare (because of the scattering of light) is a common complaint. The most common cause of decreased vision in persons with background retinopathy is macular edema.28,29 It represents fluid accumulation in the retina stemming from a breakdown in the blood–retina barrier. Proliferative diabetic retinopathy represents a more severe retinal change than background retinopathy. It is characterized by formation of fragile blood vessels (i.e., neovascularization) at the disk and elsewhere in the retina. These vessels grow in front of the retina along the posterior surface of the vitreous or into the vitreous. They threaten vision in two ways. First, because they are abnormal, they often bleed easily, leaking blood into the vitreous cavity and decreasing visual acuity. Second, the blood vessels attach firmly to the retinal surface and posterior surface of the vitreous, such that normal movement of the A B C D FIGURE 54-15 Diabetic retinopathy. (A) View of the ocular fundus in a patient with background diabetic retinopathy. Several yellowish “hard” exudates, which are rich in lipids, are evident, together with several relatively small retinal hemorrhages. (B) A vascular frond has extended anterior to the retina in the eye with proliferative retinopathy. (C) Numerous microaneurysms are present in this flat preparation of a diabetic retina. (D) This flat preparation from a diabetic is stained with periodic acid–Schiff (PAS) after the retinal vessels had been perfused with India ink. Microaneurysms (arrows) and an exudate (arrowhead) are evident in a region of retinal nonperfusion. (Rubin E., Farber J.L. [1999]. Pathology [3rd ed., p. 1553]. Philadelphia: Lippincott-Raven) CHAPTER 54 vitreous may exert a pull on the retina, causing retinal detachment and progressive blindness. Because early proliferative diabetic retinopathy is likely to be asymptomatic, it must be identified early, before bleeding occurs and obscures light transmission or leads to fibrosis and retinal detachment. The cause of diabetic retinopathy is uncertain. Many studies have demonstrated that hyperglycemia, hypertension, and hyperlipidemia contribute to the pathogenesis of the disorder.30 Either directly or indirectly, these conditions are thought to contribute to the growth of abnormal retinal blood vessels secondary to ischemia. These blood vessels grow in an attempt to supply oxygenated blood to hypoxic retinal. It has been suggested that conditions such as leukostasis may play a role. Leukocytes have a large cell volume, high cytoplasmic rigidity, a natural tendency to adhere to the vascular endothelium, and a capacity to generate toxic superoxide radicals and proteolytic enzymes. In diabetics, the leukocytes tend to be less deformable, and a greater number are activated, indicating that they may be involved in retinal capillary underperfusion, endothelial cell damage, and vascular leakage.28,29 As a result of the occluded capillaries, retinal ischemia stimulates a pathologic neovascularization mediated by angiogenic factors such as VEGF. Whereas leukocytes have been implicated in the pathogenesis of diabetic retinopathy, other blood components such as platelets and red blood cells are probably also involved. The American Diabetes Association, American College of Physicians, and American Academy of Ophthalmology have developed screening guidelines for diabetic retinopathy.30 These guidelines recommend that persons with type 1 diabetes should have an initial dilated and comprehensive eye examination by an ophthalmologist or optometrist within 3 to 5 years after the onset of diabetes. Usually, screening is not indicated before the start of puberty. Persons with type 2 diabetes should have an initial comprehensive examination shortly after diagnosis. Subsequent examinations for persons with either type 1 or type 2 diabetes should be repeated annually. The ocular examination by the ophthalmologist should include acuity measurements, slit-lamp biomicroscopy, and direct and indirect ophthalmoscopy of the retina through fully dilated pupils. When indicated, color fundus photographs and fluorescein angiograms should be done. When planning pregnancy, women with preexisting diabetes should be counseled about the risk for developing retinopathy or progression of existing retinopathy. Women who become pregnant should have a comprehensive eye examination just before or soon after conception and at least every 3 months throughout pregnancy. Preventing diabetic retinopathy from developing or progressing is considered the best approach to preserving vision. Growing evidence suggests that careful control of blood glucose levels in persons with diabetes mellitus may retard the onset and progression of retinopathy. The Diabetes Control and Complications Trial Research Group demonstrated that intensive management of persons with type 1 diabetes to maintain blood glucose levels at nearnormal levels reduced the risk for retinopathy by 76% in Disorders of Visual Function 1315 persons with no retinopathy and slowed the progress by 54% in persons with early disease.33 There also is need for intensive management of hypertension34 and hyperlipidemia, both which have been shown to increase the risk for diabetic retinopathy.30 Photocoagulation using an argon laser provides the major direct treatment modality for diabetic retinopathy.31,32 Treatment strategies include laser photocoagulation applied directly to leaking microaneurysms and grid photocoagulation with a checkerboard pattern of laser burns applied to diffuse areas of leakage and thickening.31 Because laser photocoagulation destroys the proliferating vessels and the ischemic retina, it reduces the stimulus for further neovascularization. However, photocoagulation of neovascularization near the disk is not recommended.31 Vitrectomy has proved effective in removing vitreous hemorrhage and severing vitreoretinal membranes that develop. Because of the limitations of current treatment measures, new pharmacologic therapies are being developed, targeting the underlying biochemical mechanisms that cause diabetic retinopathy. Among the pharmacologic therapies being explored are those related to the control of the growth factors and signaling systems that participate in the abnormal proliferation of retinal vessels.28,29 Hypertensive Retinopathy. Long-standing systemic hypertension results in the compensatory thickening of arteriolar walls, which effectively reduces capillary perfusion pressure. Ordinarily, a retinal blood vessel is transparent and seen as a red line; in venules, the red cells resemble a string of boxcars. On ophthalmoscopy, arteries in persons with longstanding hypertension appear paler than veins because they have thicker walls. The thickened arterioles in chronic hypertension become opaque and have a copper-wiring appearance. Edema, microaneurysms, intraretinal hemorrhages, exudates, and cotton-wool spots all are observed (see Fig. 54-12). Malignant hypertension involves swelling of the optic disk because of the local edema produced by escaped fluid. If the condition is permitted to progress long enough, serious visual deficits result. Protective thickening of arteriolar walls cannot occur with sudden increases in blood pressure. Therefore, hemorrhage is likely to occur. Trauma to the optic globe or the head, sudden high blood pressure in preeclampsia, and some types of renal disease may be accompanied by edema of the retina and optic disk and by an increased likelihood of hemorrhage. Atherosclerosis of Retinal Vessels. In atherosclerosis, the lumen of the arterioles becomes narrowed. As a result, the retinal arteries become tortuous and narrowed. At sites where the arteries cross and compress veins, the red cell column of the vein appears distended. Exudate accumulates on arteriolar walls as “fluffy” white plaques (cottonwool patches). These patches are damaged axons that on cross-section resemble crystalloid bodies. Deep and superficial hemorrhages are common. Atheromatous plaques of the central artery are associated with increased danger of stasis, thrombi of the central veins, and occlusion. 1316 UNIT XIII Special Sensory Function Retinal Detachment Retinal detachment involves the separation of the sensory retina from the pigment epithelium (Fig. 54-16). It occurs when traction on the inner sensory layer or a tear in this layer allows fluid, usually vitreous, to accumulate between the two layers.31 Retinal detachment that results from breaks in the sensory layer of the retina is called rhegmatogenous detachment (rhegma in Greek, meaning “rent” or “hole”). The vitreous normally adheres to the retina at the optic disk, macula, and periphery of the retina. When the vitreous shrinks, it separates from the retina at the posterior pole of the eye (posterior vitreous detachment). However, at the periphery, the vitreous pulls on the attached retina, which can lead to tearing of the retina. Vitreous fluid can enter the tear and contribute to further separation of the retina from its overlying pigment layer. Persons with high grades of myopia may have abnormalities in the peripheral retina that predispose to sudden detachment. Intraocular surgery such as cataract extraction may produce traction on the peripheral retina that causes eventual detachment months or even years after surgery. Detachment may result from exudates that separate the two retinal layers. Exudative retinal detachment may be caused by intraocular inflammation, intraocular tumors, or certain systemic diseases. Inflammatory processes include posterior scleritis, uveitis, or parasitic invasion. Retinal detachment also can follow trauma immediately or at some later time. Detachment of the neural retina from the retinal pigment layer separates the receptors from their major blood supply, the choroid. If retinal detachment continues for some time, permanent destruction and blindness of that part of the retina occur. The bipolar and ganglion cells may survive because their blood supply, by way of the retinal arteries, remains intact. Without receptors, however, there is no visual function. The primary symptom of retinal detachment is loss of vision. Sometimes, flashing lights or sparks, followed by small floaters or spots in the field of vision, occur as the retina pulls away from the posterior pole of the eye. No pain is associated with this retinal detachment. As de- Detached retina Retinal tear FIGURE 54-16 Detached retina. tachment progresses, the person perceives a dark curtain progressing across the visual field. Because the process begins in the periphery and spreads circumferentially and posteriorly, initial visual disturbances may involve only one quadrant of the visual field. Large peripheral detachments may occur without involvement of the macula, so that visual acuity remains unaffected. The tendency, however, is for detachments to enlarge until the entire retina is detached. Diagnosis is based on the ophthalmoscopic appearance of the retina. Treatment is aimed at closing retinal tears and reattaching the retina. Rhegmatogenous detachment usually requires surgical treatment. Scleral buckling or pneumatic retinopexy is the most commonly used surgical technique.31 Scleral buckling is the primary surgical procedure performed to reattach the retina. The procedure requires careful location of the retinal break and treatment with diathermy, cryotherapy, or laser to produce chorioretinal adhesions that seal the retinal tears so that the vitreous can no longer leak into the subretinal space. With scleral buckling, a piece of silicone (i.e., the buckle) is sutured and infolded into the sclera, physically indenting the sclera so that it contacts the separated pigment and retinal layers. Pneumatic retinopexy involves the intraocular injection of an expandable gas instead of a piece of silicone to form the indentation. An overall reattachment rate of 90% is reported, but the visual results depend on the preoperative status of the macula.31 The most common cause of failure after surgical treatment for detached retina is the development of membranes on the retina. Macular Degeneration Macular degeneration is characterized as loss of central vision due to destructive changes of the yellow-pigmented area surrounding the central fovea. Age-related macular degeneration is the most common cause of reduced vision in the United States. It is the leading cause of blindness among persons older than 75 years and of newly reported cases of blindness among those older than 65 years of age.1 The cause of age-related macular degeneration is poorly understood. In addition to older age, identifiable risk factors include female sex, white race, cigarette smoking, and low dietary intake of carotenoids. Increasing evidence suggests that genetic factors may also play a role.35 There are two types of age-related macular degeneration: an atrophic nonexudative or “dry” form and an exudative or “wet” form. Both types are progressive, but differ in manifestations, prognosis, and management. Although most people with age-related macular degeneration manifest nonproliferative changes only, most people who experience severe vision loss do so from the development of the exudative form of the disease.31 Nonexudative age-related macular degeneration is characterized by various degrees of atrophy and degeneration of the outer retina, Bruch’s membrane, and the choriocapillaris. It does not involve leakage of blood or serum; hence, it is called dry age-related macular degeneration. On ophthalmoscopic examination, there are visible changes in the retinal pigmentary epithelium and pale yellow spots, called drusen, that may occur individually or in groups CHAPTER 54 throughout the macula. Histopathologically, most drusen contain remnants of materials representative of focal detachment of the pigment epithelium. With time, the drusen enlarge, coalesce, and increase in number. The level of associated visual impairment is variable and may be minimal. Most people with macular drusen do not experience significant loss of central vision; and the atrophic changes may stabilize or progress slowly. However, people with the nonexudative form of age-related macular degeneration need to be followed closely because the exudative stage may develop suddenly at any time. The exudative form of macular degeneration is characterized by the formation of a choroidal neovascular membrane that separates the pigmented epithelium from the neuroretina. These new blood vessels have weaker walls than normal and are prone to leakage; therefore, this condition is called wet age-related macular degeneration. The leakage of serous or hemorrhagic fluid into the subretinal space causes separation of the pigmented epithelium from the neurosensory retina. Even though some subretinal neovascular membranes may regress spontaneously, the natural course of exudative macular degeneration is toward irreversible loss of central vision. Persons with late-stage disease often find it difficult to see at long distances (e.g., in driving), do close work (e.g., reading), see faces clearly, or distinguish colors. However, they may not be severely incapacitated because the peripheral retinal function usually remains intact. With the help of low-vision aids, many of them are able to continue many of their normal activities. The early stages of subretinal neovascularization may be difficult to detect ophthalmoscopically. Therefore, there is need to be alert for recent or sudden changes in central vision, blurred vision, or scotoma in persons with evidence of age-related macular degeneration. Although there is no treatment for the nonexudative form of macular degeneration, argon laser photocoagulation may be useful in treating the neovascularization that occurs with the exudative form.36,37 Another method used to halt neovascularization is photodynamic therapy. It is a nonthermal process leading to localized production of reactive oxygen species that mediate cellular, vascular, and immunologic injury and destruction of new blood vessels. There has been recent interest in the effect of supplemental antioxidants (high-dose vitamin C, vitamin E, and betacarotene) and zinc for persons at risk for developing macular degeneration.38 While rare, macular degeneration can occur as a hereditary condition in young persons and sometimes in adults. The genetic form of macular dystrophy, Stargardt’s disease, becomes manifest in the middle of the first to second decade of life and is inherited as a classic recessive trait, requiring mutations in both alleles of a single disease-related gene.39 The gene codes for an ABCR (ATP-binding cassette transporter—retina) transporter molecule located in the retina that functions in the transport of retinal lipids and peptides. It is thought that mutations in this gene allow degraded material to accumulate and interfere with retinal function. Disorders of Visual Function 1317 In summary, the retina covers the inner aspect of the posterior two thirds of the eyeball and is continuous with the optic nerve. It contains the neural receptors for vision, and it is here that light energy of different frequencies and intensities is converted to graded local potentials, which then are converted to action potentials and transmitted to visual centers in the brain. The photoreceptors normally shed portions of their outer segments. These segments are phagocytized by cells in the pigment epithelium. Failure of phagocytosis, as occurs in one form of retinitis pigmentosa, results in degeneration of the pigment layer and blindness. The retina receives its blood from two sources: the choriocapillaris, which supplies the pigment layer and the outer portion of the sensory retina adjacent to the choroid, and the branches of the retinal artery, which supply the inner half of the retina. Retinal blood vessels are normally apparent through the ophthalmoscope. Disorders of retinal vessels can result from many local and systemic disorders, including diabetes mellitus and hypertension. They cause vision loss through changes that result in hemorrhage, production of opacities, and separation of the pigment epithelium and sensory retina. Retinal detachment involves separation of the sensory receptors from their blood supply; it causes blindness unless reattachment is accomplished promptly. Macular degeneration is characterized as loss of central vision due to destructive changes of the yellow-pigmented area surrounding the central fovea. Agerelated macular degeneration is the most common cause of reduced vision in the United States. Disorders of Neural Pathways and Cortical Centers After completing this section of the chapter, you should be able to meet the following objectives: ✦ Characterize what is meant by a visual field defect ✦ Explain the use of perimetry in the diagnosis of a visual field defect ✦ Define the terms hemianopia, quadrantanopia, heteronymous hemianopia, and homonymous hemianopia and relate to disorders of the optic pathways ✦ Describe visual defects associated with disorders of the visual cortex and visual association areas Full visual function requires the normally developed brain-related functions of photoreception and the pupillary reflex. These functions depend on the integrity of all visual pathways, including retinal circuitry and the pathway from the optic nerve to the visual cortex and other visual regions of the brain and brain stem. OPTIC PATHWAYS Visual information is carried to the brain by axons of the retinal ganglion cells, which form the optic nerve. Sur- 1318 UNIT XIII Special Sensory Function Left visual field Temporal Right visual field Nasal Temporal Whitened field no vision Right eye Left eye Left Right Lesion 1 Optic nerve Optic tract 1 Right optic nerve 2 3 Lesion 2 Optic chiasm Lesion 3 Optic radiation Right optic tract rounded by pia mater, cerebrospinal fluid (CSF), arachnoid, and the dura mater, the optic nerve represents an outgrowth of the brain rather than a peripheral nerve. The optic nerve extends from the back of the optic globe through the orbit and the optic foramen, into the middle fossa, and on to the optic chiasm at the base of the brain11 (Fig. 54-17). Axons from the nasal portion of the retina remain medial, and those from the temporal retina remain lateral in the optic nerve. The two optic nerves meet and fuse in the optic chiasm, beyond which they are continued as the optic tracts. In the optic chiasm, axons from the nasal retina of each eye cross to the opposite side and join with the axons of the temporal retina of the contralateral eye to form the optic tracts. Thus, one optic tract contains fibers from both eyes that transmit information from the same visual field. VISUAL CORTEX The primary visual cortex (area 17) surrounds the calcarine fissure, which lies in the occipital lobe. It is at this level that visual sensation is first experienced (Fig. 54-18). Immediately surrounding area 17 are the visual association cortices (areas 18 and 19) and several other association cortices.11 These association cortices, with their thalamic nuclei, must be functional for added meaningfulness of visual perception. This higher-order aspect of the visual experience depends on previous learning. Circuitry in the primary visual cortex and the visual association areas is extremely discrete with respect to the location of retinal stimulation. For example, specific neurons respond to the particular orientation of a moving edge, specific colors, or familiar shapes. This elaborate organization of the visual cortex, with its functionally separate and multiple representations of the same visual field, FIGURE 54-17 Diagram of optic pathways. The red lines indicate the right visual field and the blue lines the left visual field. Note the crossing of fibers from the medial half of each retina at the optic chiasm. Lesion 1 (right optic nerve) produces unilateral blindness. Lesion 2 (optic chiasm) may involve only those fibers that originate in the nasal half of each retina and cross to the opposite side in the optic chiasm; visual loss involves the temporal half of each field (bitemporal hemianopia). Lesion 3 (right optic tract) interrupts fibers (and vision) originating on the same side of both eyes (homonymous) with loss of vision from half of each field (hemianopia). provides the major basis for visual sensation and perception. Because of this discrete circuitry, lesions of the visual cortex must be large to be detected clinically. VISUAL FIELDS The visual field refers to the area that is visible during fixation of vision in one direction. Because visual system deficits are often expressed as visual field deficits rather than as direct measures of neural function, the terminology for normal and abnormal visual characteristics usually is based on visual field orientation. Most of the visual field is binocular, or seen by both eyes. This binocular field is subdivided into central and pe- Frontal eye field (part of 8) Somatosensory (3, 1, 2) Somatosensory association (5, 7) Visual (17) (19) (18) Second somatosensory Auditory (41) Auditory association (42, 22) Visual association FIGURE 54-18 Lateral view of the cortex illustrating the location of the visual, visual association, auditory, and auditory association areas. CHAPTER 54 ripheral portions. Central portions of the retina provide high visual acuity and correspond to the field focused on the central fovea; the peripheral and surrounding portion provides the capacity to detect objects, particularly moving objects. Beyond the visual field shared by both eyes, the left lateral periphery of the visual field is seen exclusively by the left nasal retina, and the right peripheral field is seen by the right nasal retina. As with a camera, the simple lens system of the eye inverts the image of the external world on each retina. In addition, the right and left sides of the visual field also are reversed. The right binocular visual field is seen by the left retinal halves of each eye: the nasal half of the right eye and the temporal half of the left eye. Once the level of the retina is reached, the nervous system plays a consistent role. The upper half of the visual field is received by the lower half of the retinas of both eyes. Representations of this upper half of the field are carried in the lower half of each optic nerve: they synapse in the lower half of the lateral geniculate nucleus (LGN) of each side of the brain. Neurons in this part of the LGN send their axons through the inferior half of the optic radiation, looping into the temporal lobe to terminate in the lower half of the primary visual cortex on each side of the brain. Because of the lateral separation of the two eyes, each eye contributes a different image of the world to the visual field. This is called binocular disparity. Disparity between the laterally displaced images seen by the two eyes provides a powerful source of three-dimensional depth perception for objects within a distance of 30 m. Beyond that distance, binocular disparity becomes insignificant: depth perception is based on other cues (e.g., the superimposition of the image of near objects over that of far objects, and the faster movement of near objects than of far objects). Visual Field Defects Visual field defects result from damage to the visual pathways or the visual cortex. Perimetry or visual field testing, in which the visual field of each eye is measured and plotted in an arc, is used to identify defects and determine the location of lesions. Retinal Defects. All of us possess a hole, or scotoma, in our visual field, of which we are unaware. Because the optic disk, where the optic nerve fibers exit the retina, does not contain photoreceptors, the corresponding location in the visual field constitutes a blind spot. Local retinal damage caused by small vascular lesions (i.e., retinal stroke) and other localized pathologies can produce additional blind spots. As with the normal blind spot, persons are usually not aware of the existence of scotomata in their visual fields unless they encounter problems seeing objects in certain restricted parts of the visual field. Absences near or in the center of the bilateral visual field can be annoying and even disastrous. Although the hole is not recognized as such, the person finds that a part of a printed page appears or disappears, depending on where the fixation point is held. Most persons learn to position their eyes to use the remaining central foveal vision for high-acuity tasks. Defects in the peripheral visual field, including the monocular peripheral fields, are less annoy- Disorders of Visual Function 1319 ing but potentially more dangerous. The person who is unaware of the defect, when walking or driving an automobile, does not see cars or bicyclists until their image reaches the functional visual field—sometimes too late to avert an accident. With careful education, a person can learn to shift the gaze constantly to obtain visual coverage of important parts of the visual field. If the damage is at the retinal or optic nerve level, only the monocular field of the damaged eye becomes a problem. A lesion affecting the central foveal vision of one eye can result in complaints of eyestrain during reading and other close work because only one eye really is being used. Localized damage to the optic tracts, LGN, optic radiation, or primary visual cortex affects corresponding parts of the visual fields of both eyes. Disorders of the Optic Pathways The visual pathway extends from the front to the back of the head. It is much like a telephone line between distant points in that damage at any point along the pathway results in functional defects (see Fig. 54-17). Among the disorders that can interrupt the visual pathway are vascular lesions, trauma, and tumors. For example, normal visual system function depends on vascular adequacy in the ophthalmic artery and its branches; the central artery of the retina; the anterior and middle cerebral arteries, which supply the intracranial optic nerve, chiasm, and optic tracts; and the posterior cerebral artery, which supplies the LGN, optic radiation, and visual cortex. The adequacy of posterior cerebral artery function depends on that of the vertebral and basilar arteries that supply the brain stem. Vascular insufficiency in any one of these arterial systems can seriously affect vision. Examination of visual system function is of particular diagnostic use because lesions at various points along the pathway have characteristic symptoms that assist in the localization of the pathology. Visual field defects of each eye and of the two eyes together are useful in localizing lesions affecting the system. Blindness in one eye is called anopia. If half of the visual field for one eye is lost, the defect is called hemianopia; loss of a quarter field is called quadrantanopia. Enlarging pituitary tumors can produce longitudinal damage through the optic chiasm with loss of the medial fibers of the optic nerve representing both nasal retinas and both temporal visual half-fields. Loss of the temporal or peripheral visual fields on both sides results in a narrow binocular field, commonly called tunnel vision. The loss of different halffields in the two eyes is called a heteronymous loss, and the abnormality is called heteronymous hemianopia. Destruction of one or both lateral halves of the chiasm is common with multiple aneurysms of the circle of Willis. In this condition, the function of one or both temporal retinas is lost, and the nasal fields of one or both eyes are lost. The loss of the temporal fields (nasal retina) of both eyes is called bitemporal heteronymous anopia. With both eyes open, the person with bilateral defects still has the full binocular visual field. Loss of the optic tract, LGN, full optic radiation, or complete visual cortex on one side results in loss of the corresponding visual half-fields in each eye. Homonymous means 1320 UNIT XIII Special Sensory Function “the same” for both eyes. In left-side lesions, the right visual field is lost for each eye and is called complete right homonymous hemianopia. Partial injury to the left optic tract, LGN, or optic radiation can result in the loss of one fourth of the visual field in both eyes. This is called homonymous quadrantanopia, and depending on the lesion, it can involve the upper (superior) or lower (inferior) fields. Because the optic radiation fibers for the superior fourth of the visual field traverse the temporal lobe, superior quadrantanopia is more common. The LGN, optic radiation, and visual cortex all receive their major blood supply from the posterior cerebral artery; unilateral occlusion of this artery results in complete loss of the opposite field (i.e., homonymous hemianopia). Bilateral occlusion of these arteries results in total cortical blindness. Disorders of the Visual Cortex Discrete damage to the binocular portion of the primary visual cortex also can result in scotomata in the corresponding visual fields. If the visual loss is in the central high-acuity part of the field, severe loss of visual acuity and pattern discrimination occurs. The central high-acuity portion of the visual field is located at the occipital pole. This region can be momentarily compressed against the occipital bone (i.e., contrecoup) after severe trauma to the frontal part of the cranium. Mechanical trauma to the cortex results in firing of neurons, experienced as flashes of light or “seeing stars.” Destruction of the polar visual cortex causes severe loss of visual acuity and pattern discrimination. Such damage is permanent and cannot be corrected with lenses. The bilateral loss of the entire primary visual cortex, called cortical blindness, eliminates all visual experience. Crude analysis of visual stimulation at reflex levels, such as eye-orienting and head-orienting responses to bright moving lights, pupillary reflexes, and blinking at sudden bright lights, may be retained even though vision has been lost. Extensive damage to the visual association cortex (areas 18 and 19) that surrounds an intact primary visual cortex results in a loss of the learned meaningfulness of visual images (i.e., visual agnosia). The patient can see the patterns of color, shapes, and movement, but no longer can recognize formerly meaningful stimuli. Familiar objects can be described but not named or reacted to meaningfully. However, if other sensory modalities, such as hearing and touch, can be applied, full recognition occurs. This disorder represents a problem of recognition rather than intellect. Testing of Visual Fields Crude testing of the binocular visual field and the visual field of each individual eye (i.e., monocular vision) can be accomplished without specialized equipment. In the confrontation method, the examiner stands or sits 2 to 3 feet in front of the person to be tested and instructs the person to focus on an object such as a penlight with one eye closed. The object is moved from the center toward the periphery of the person’s visual field and from the periphery toward the center, and the person is instructed to report the presence or absence of the object. By moving the ob- ject through the vertical, horizontal, and oblique aspects of the visual field, a crude estimate can be made of the visual field. Large field defects can be estimated by the confrontation method, and it may be the only way for testing young children and uncooperative adults. Accurate determination of the presence, size, and shape of smaller holes, or scotomata, in the visual field of a particular eye can be demonstrated only by perimetry. This is done by having the person look with one eye toward a central spot directly in front of the eye while the head is stabilized by a chin rest or bite board. A small dot of light or a colored object is moved back and forth in all areas of the visual field. The person reports whether the stimulus is visible and, if a colored stimulus is used, what the perceived color is. A hemispheric support is used to control and standardize the movement of the test object, and a plot of radial coordinates of the visual field is made. Perimetry provides a means of determining alterations from normal and, with repeated testing, a way of following the progress of the disease or treatment. In summary, visual information is carried to the brain by axons of the retinal ganglion cells that form the optic nerve. The two optic nerves meet and fuse in the optic chiasm. The axons of each nasal retina cross in the chiasm and join the uncrossed fibers of the temporal retina of the opposite eye in the optic tract to form the optic tracts. The fibers of each optic tract then synapse in the LGN, and from there, travel by way of the optic radiations to the primary visual cortex in the calcarine area of the occipital lobe. Damage to the visual pathways or visual cortex leads to visual field defects that can be identified through visual field testing or perimetry and used to determine the lesion’s location. Damage to the visual association cortex can result in the phenomenon of seeing an object, but with loss of learned recognition (i.e., visual agnosia). Disorders of Eye Movement After completing this section of the chapter, you should be able to meet the following objectives: ✦ Describe the function and innervation of the extraocular muscles ✦ Recognize the use of smooth pursuit, saccadic, and vergence conjugate gaze movements in self or others ✦ Cite the function of optic tremor in terms of visual function ✦ Explain the difference between paralytic and nonparalytic strabismus ✦ Define amblyopia and explain its pathogenesis ✦ Explain the need for early diagnosis and treatment of eye movement disorders in children Normal vision depends on the coordinated action of the entire visual system and a number of central control systems. It is through these mechanisms that an object is simultaneously imaged on the fovea of both eyes and perceived as a single image. Strabismus and amblyopia are CHAPTER 54 two disorders that affect this highly integrated system. Although strabismus may develop in later life, it is seen most commonly in children, among whom its incidence is approximately 4%.40 EXTRAOCULAR EYE MUSCLES AND THEIR INNERVATION Each eyeball can rotate around its vertical axis (lateral or medial rotation in which the pupil moves away from or toward the nose), its horizontal left-to-right axis (vertical elevation or depression, in which the pupil moves up or down), and longitudinal horizontal axis in which the top of the pupil moves toward or away from the nose. Three pairs of extraocular muscles—the superior and inferior recti, the medial and lateral recti, and the superior and inferior obliques—control the movement of each eye (Fig. 54-19). The four rectus muscles are named according to where they insert into the sclera on the medial, lateral, inferior, and superior surfaces of each eye. The two oblique muscles insert on the lateral posterior quadrant of the eyeball—the superior oblique on the upper surface and the inferior oblique on the lower. Each of the three sets of muscles in each eye is reciprocally innervated so that one muscle relaxes when the other contracts. Reciprocal contraction of the medial and lateral recti moves the eye from side to side (adduction and abduction); the superior and inferior recti move the eye up and down (elevation and depression). The oblique muscles rotate (intorsion and extorsion) the eye around its optic axis. A seventh muscle, the levator palpebrae superioris, elevates the upper lid. The extraocular muscles are innervated by three cranial nerves. The abducens nerve (CN VI) innervates the lateral rectus, the trochlear nerve (CN IV) innervates the superior oblique, and the oculomotor nerve (CN III) innervates the remaining four muscles. The extraocular muscles are among the most rapidly contracting and precisely controlled skeletal muscles in the entire body. This reflects the high nerve-to-muscle ratio, with one lower motoneu- Disorders of Visual Function ron (LMN) innervating 2 to 10 muscle fibers. Table 54-1 describes the function and innervation of the extraocular muscles. The CN VI (abducens) nucleus, in the caudal pons, innervates the lateral rectus muscle, which rotates the ipsilateral (same side) eye laterally (abduction). The long pathway of CN VI along the floor of the cranial cavity from the pons to the orbit makes it vulnerable to damage from severe injury or fracture of the cranial base. Partial or complete damage to this nerve results in weakness or complete paralysis of the muscle. Medial gaze is normal, but the affected eye fails to rotate laterally with an attempted gaze toward the affected side, a condition called medial strabismus. The CN IV (trochlear) nucleus, at the junction of the pons and midbrain, innervates the contralateral or opposite side superior oblique muscle, which rotates the top of the globe inward toward the nose, a movement called intorsion. In combination with other muscles, it also contributes strength to movement of the innervated eye downward and inward. The superior oblique muscle neurons cross over the roof of the midbrain, drop vertically through the roof of the cavernous sinus, and then enter the orbit. This short pathway is rarely damaged, and signs of dysfunction are usually the result of a small brain stem stroke affecting the CN IV nucleus or from damage to the midbrain-level vertical gaze networks. The CN III (oculomotor) nucleus, which extends through a considerable part of the midbrain, contains clusters of LMNs for each of the five eye muscles it innervates: inferior rectus, ipsilateral superior rectus, inferior oblique, medial rectus, and levator palpebrae superioris. The medial rectus, superior rectus, and inferior rectus rotate the eye in the directions shown in Table 54-1. The action of the inferior rectus is antagonistic to the superior rectus. Because of its plane of attachment to the globe, the inferior oblique rotates the eye in the frontal plane (i.e., torsion), pulling the top of the eye laterally (i.e., extorsion). The levator palpebrae superioris elevates the upper lid and is involved only in vertical gaze eye movements. As the eyes rotate upward, the upper lid is reflexively retracted, and in Inferior oblique Medial rectus Temporalis muscle Lateral rectus Superior oblique Levator palpebrae superioris Superior rectus Medial rectus Superior oblique Inferior rectus Superior rectus Stump of levator palpebrae Optic nerve FIGURE 54-19 Extraocular eye muscles of the right eye. 1321 Inferior rectus Lateral rectus Inferior oblique 1322 UNIT XIII Special Sensory Function 3–IO SR–3 6–LR TABLE 54-1 MR MR 3 3 IR–3 4–SO 3–SR 3–IR IO–3 LR–6 SO–4 Eye in Primary Position: Extrinsic Ocular Muscle Actions Muscle* Innervation Primary Secondary Tertiary MR: medial rectus LR: lateral rectus SR: superior rectus IR: inferior rectus SO: superior oblique IO: inferior oblique III VI III III IV III Adduction Abduction Elevation Depression Intorsion Extorsion Intorsion Extorsion Depression Elevation Adduction Adduction Abduction Abduction *In the schema of the functional roles of the six extraocular muscles, the major directional force applied by each muscle is indicated on the top. These muscles are arranged in functionally opposing pairs per eye and in parallel opposing pairs for conjugate movements of the two eyes. The numbers associated with each muscle indicate the cranial nerve innervation: 3, oculomotor (III) cranial nerve; 4, trochlear (IV) cranial nerve; 6, abducens (VI) cranial nerve. the downward gaze, the upper lid is lowered, restricting the exposure of the conjunctiva to air and reducing the effects of drying. Eye movements include lateral gaze, vertical gaze, oblique gaze, and torsional gaze. Lateral gaze movements are in the horizontal plane and are controlled by lateral gaze centers. These gaze centers consist of interneurons in the abducens (CN VI) nucleus on the side of abduction. Vertical gaze upward or downward is controlled by a vertical gaze center of interneurons in and near the oculomotor (CN III) nucleus. Oblique gaze involves a combination of lateral and vertical gaze controls. Torsional gaze movements involve ocular rotation around the optical axis of the eyes, and although they occur frequently, they are more difficult to observe. The torsional gaze center involves interneurons in the bilaterally located trochlear (CN IV) nucleus on both sides. Each of these control centers maintains a moderate level of tonic activity (i.e., low level of “spontaneous” action potentials) in each opposing pair of muscles. This maintains a neutral position of eye posture. More motor units are automatically recruited when an eye deviates from this neutral position. When released from a directional signal, the eyes automatically return to the neutral position. Communication between the eye muscle nuclei of each side occurs primarily through the posterior commissure at the rostral end of the midbrain. Longitudinal communication among the three nuclei occurs along a fiber tract called the medial longitudinal fasciculus (MLF), which extends from the midbrain to the upper part of the spinal cord. Each pair of eye muscles is reciprocally innervated, by way of the MLF or other associated pathways, so that as one muscle contracts, the other relaxes. For example, the LMNs of CN VI produce lateral rotation (i.e., abduction) of the left eye. Simultaneously, interneurons of the left CN VI, which communicate with the right CN III by way of the MLF, move the right eye medially (adduction). These MLFlinked communication paths are vulnerable to damage in the caudal midbrain and pons. Damage to the pontine MLF on one side results in a loss of this linkage such that lateral deviation in the ipsilateral eye no longer is linked to adduction on the contralateral side (i.e., internuclear ophthalmoplegia). If the MLF is damaged bilaterally, the linkage is lost for lateral gaze in either direction. Eye Movements and Gaze Conjugate movements are those in which the optical axes of the two eyes are kept parallel, sharing the same visual field. Gaze refers to the act of looking steadily in one direction. Eye movements can be categorized into five classes of movements: smooth pursuit movements, saccadic movements, optic tremor, vergence movement, and nystagmus. Although the conjugate reflexes are essential to efficient visual function during head movement or target movement, their circuitry is so deeply embedded in CNS function that they are present and can be elicited when the eyes are closed, during sleep, and in deep coma, and they function normally and accurately in congenitally blind persons. Smooth Pursuit Movements. Smooth pursuit movements are tracking movements that serve to maintain an object at a fixed point in the center of the visual fields of both eyes. The object may be moving and the eyes following, or the object may be stationary and the head of the observer moving. Normal eye posture is a conjugate gaze directed straight forward with the head held in a forward-looking posture. Smooth pursuit movements normally begin from this position. In fact, holding a strongly deviated gaze becomes tiring after about 30 seconds, and most people will CHAPTER 54 make head and body rotation adjustments to bring the eyes to a central position within that time. Voluntary pursuit movements are tested by asking the person to follow a finger or another object as it is moved slowly through the visual field. Successful conjugate following requires a functional optic system communicating to the superior colliculus and to the primary visual cortex. Saccadic Eye Movements. Saccadic eye movements are sudden, jerky conjugate movements that quickly change the fixation point. During reading, the fixation pattern involves a focus on a word or short series of words and then a sudden jump of the eyes to a new fixation point on the next word or phrase. These shifts in fixation points are saccadic movements. During the saccade, a person does not experience the blur of the rapidly moving visual field. The mechanism by which visual experience is momentarily shut off is not understood. Saccadic movements are automatic reflex movements that, in most situations, operate at the brain stem level. Saccadic shifts of a gaze toward the source of a sudden, unexpected visual, auditory, tactile, or painful stimulus are a component of the startle pattern. This is functionally a visual grasping reflex, redirecting conjugate gaze in the direction of the startle stimulus. This reflex occurs in the absence of the cortical portion of the visual system (cortical blindness). The auditory startle reaction (startle reflex) involves rapid saccadic movements in the direction of a auditory stimulus. It is present in the neonate and in persons with impaired cortical auditory apparatus. The frontal eye fields of the premotor cortex are important for voluntary saccadic movements such as reading. If this frontal premotor area is not functional, a person can describe objects in the visual field but cannot voluntarily search the visual environment. Disorders of Visual Function 1323 STRABISMUS Strabismus, or squint, refers to any abnormality of eye coordination or alignment that results in loss of binocular vision (Fig. 54-20). When images from the same spots in visual space do not fall on corresponding points of the two retinas, diplopia, or double vision, occurs. In standard terminology, the disorders of eye movement are described according to the direction of movement. Esotropia refers to medial deviation, exotropia refers to lateral deviation, hypertropia refers to upward deviation, hypotropia refers to downward deviation, and cyclotropia refers to torsional deviation. The term concomitance refers to equal deviation in all directions of gaze. A nonconcomitant strabismus is one that varies with the direction of gaze. Strabismus may be divided into paralytic (nonconcomitant) forms, in which there is weakness or paralysis of one or more of the extraocular muscles, and nonparalytic (concomitant) forms, in which there is no primary muscle impairment. Strabismus is called intermittent, or periodic, when there are periods in which the eyes are parallel. It is monocular when the same eye always deviates and the fellow eye fixates. Figure 54-21 illustrates abnormalities in eye movement associated with esotropia and exotropia. Strabismus affects approximately 4% of children younger than 6 years of age.40,41 Because 30% to 50% of these children sustain permanent secondary loss of vision, or amblyopia, if the condition is left untreated, early diagnosis and treatment are essential.42,43 Eye Tremor. An eye tremor refers to involuntary, rhythmic, oscillatory eye movements, occurring approximately 10 times per second. Small-range optical tremors are a normal and useful independent function of each eye. One function of the fine optic tremor is to constantly move a bright image onto a new bank of cones, permitting previously stimulated receptors quickly to recover from adaptation. Vergence Eye Movements. Vergence movements are those that move the eyes in opposite directions to keep the image of an object precisely positioned on the fovea of each eye. Convergence and divergence, which assist in maintaining a binocularly fixed image in near vision, have a major role in accurate depth perception. The vergence system is driven by retinal disparity (i.e., differential placement of an object’s image on each retina). A nearby target (<30 feet) moving in the same dimension as the optical axis elicits a reflex mechanism that provides redirection of the optical axes of each eye away from parallel (i.e., in opposite directions) in the horizontal plane (i.e., vergence gaze). This process permits a continued binocular focus on the near target. Perception of depth is a higher-order function of the cortical visual system and is based on one or more of several classes of stimuli, such as superimposition and relative movement. FIGURE 54-20 Photograph of a child with intermittent exotropia squinting in the sunlight. (Vaughn D.G., Asbury T., Riordon-Eva P. [1995]. General ophthalmology [p. 239]. Stamford, CT: Appleton & Lange) 1324 UNIT XIII Special Sensory Function A Left gaze: no deviation B D Right hypertropia Primary position: right esotropia C Right gaze: left esotropia E Paralytic Strabismus Paralytic strabismus results from paresis (i.e., weakness) or plegia (i.e., paralysis) of one or more of the extraocular muscles. When the normal eye fixates, the affected eye is in the position of primary deviation. A manifestation of esotropia is a weakness of one of the lateral rectus muscles, usually the result of weakness of the abducens nerve (CN VI). When the affected eye fixates, the unaffected eye is in a position of secondary deviation. The secondary deviation of the unaffected eye is greater than the primary deviation of the affected eye. This is because the affected eye requires an excess of innervational impulse to maintain fixation; the excess impulses also are distributed to the unaffected eye, causing overaction of its muscles.40 Paralytic strabismus is uncommon in children but accounts for nearly all cases of adult strabismus; it can be caused by many conditions. Paralytic strabismus is seen most commonly in adults who have had cerebral vascular accidents and may occur as the first sign of a tumor or inflammatory condition involving the CNS. One type of muscular dystrophy exerts its effects on the extraocular muscles. Initially, eye movements in all directions are weak, with later progression to bilateral optic immobility. Weakness of eye movement and lid elevation is often the first evidence of myasthenia gravis. The pathway of the oculomotor (CN III), trochlear (CN IV), and abducens (CN VI) nerves through the cavernous sinus and the back of the Right exotropia FIGURE 54-21 Paralytic strabismus associated with paralysis of the right lateral rectus muscle: (A) primary position (looking straight ahead) of the eyes; (B) left gaze with no deviation; and (C) right gaze with left esotropia. (D) Primary position of the eyes with weakness of the right inferior rectus and right hypertropia; and (E) primary position of the eyes with weakness of the right medial rectus and right exotropia. orbit make them vulnerable to basal skull fracture and tumors of the cavernous sinus (e.g., cavernous sinus syndrome) or orbit (e.g., orbital syndrome).44 In infants, paralytic strabismus can be caused by birth injuries affecting the extraocular muscles or the cranial nerves supplying these muscles. It also can result from congenital anomalies of the muscles. In general, paralytic strabismus in an adult with previously normal binocular vision causes diplopia. This does not occur in persons who have never developed binocular vision. Nonparalytic Strabismus In nonparalytic strabismus, there is no extraocular muscle weakness or paralysis, and the angle of deviation is always the same in all fields of gaze. With persistent deviation, secondary abnormalities may develop because of overaction or underaction of the muscles in some fields of gaze. Nonparalytic esotropia is the most common type of strabismus. The disorder may be accommodative, nonaccommodative, or a combination of the two. Accommodative strabismus is caused by disorders such as uncorrected hyperopia, in which the esotropia occurs with accommodation. Onset of this type of esotropia characteristically occurs between 18 months and 4 years of age because accommodation is not well developed until that time. The disorder most often is monocular but may be alternating. Approximately 50% of the cases of esotropia are accom- CHAPTER 54 Disorders of Visual Function 1325 modative. The causes of nonaccommodative strabismus are obscure. This disorder may be related to faulty muscle insertion, fascial abnormalities, or faulty innervation. Research suggests that idiopathic strabismus may have a genetic basis; siblings often present with similar disorders. cal procedures may be used to strengthen or weaken an extraocular muscle by altering its length or attachment site. Diagnosis and Treatment Amblyopia describes a condition of diminished vision (uncorrectable by lenses) in which no detectable organic lesion of the eye is present.40,47,48 This condition is sometimes called lazy eye. Types of amblyopia include deprivation occlusion, strabismus, and refractive and organic amblyopia. It is caused by visual deprivation (e.g., cataracts, severe ptosis) or abnormal binocular interactions (e.g., strabismus, anisometropia) during visual immaturity. Normal development of the thalamic and cortical circuitry necessary for binocular visual perception requires simultaneous binocular use of each fovea during a critical period early in life (0 to 5 years). In infants with unilateral cataracts that are dense, central, and larger than 2 mm in diameter, this time is before 2 months of age.40 In conditions causing abnormal binocular interactions, one image is suppressed to provide clearer vision. In esotropia, vision of the deviated eye is suppressed to prevent diplopia. A similar situation exists in anisometropia, in which the refractive indexes of the two eyes are different. Although the eyes are correctly aligned, they are unable to focus together, and the image of one eye is suppressed. In animal experiments, monocular deprivation results in reduced synaptic density in the LGN and the primary visual cortical areas that process information from the affected eye or eyes.47 The reversibility of amblyopia depends on the maturity of the visual system at the time of onset and the duration of the abnormal experience. If esotropia is involved, some persons alternate eyes and do not experience diplopia. With late adolescent or adult onset, this habit pattern must be unlearned after correction. Peripheral vision is less affected than central foveal vision in amblyopia. Suppression becomes more evident with high illumination and high contrast. It is as if the affected eye did not possess central vision and the person learns to fixate with the nonfoveal retina. If bilateral congenital blindness or near blindness (e.g., from cataracts) occurs and remains uncorrected during infancy and early childhood, the person remains without pattern vision and has only overall field brightness and color discrimination. This is essentially bilateral amblyopia. The treatment of children with the potential for development of amblyopia must be instituted well before the age of 6 years to avoid the suppression phenomenon. Surgery for congenital cataracts and ptosis should be done early. Severe refractive errors should be corrected. In strabismus, alternately blocking vision in one eye and then the other forces the child to use both eyes for form discrimination. The duration of occlusion of vision in the good eye must be short (2 to 5 hours per day) and closely monitored, or deprivation amblyopia can develop in the good eye as well. Although amblyopia is not likely to occur after 8 or 9 years of age, some plasticity in central circuitry is evident even in adulthood. For example, after refractive correction for long-standing astigmatism in adults, visual All infants and children should be examined for visual alignment. Alignment of the visual axis occurs in the first 3 months of life.45 All infants should have consistent, synchronized eye movement by 5 to 6 months of age. Infants who have reached this age and whose eyes are not aligned at all times during waking hours should be examined by a qualified practitioner. Rapid assessment of extraocular muscle function is accomplished by three methods. The first occurs in a darkened room with the child staring straight ahead. A penlight is then pointed at the midpoint between the two eyes, and a bright dot of reflected light can be seen on the cornea of each eye. With normal eye alignment, the reflected light should appear at the same spot on the cornea of each eye. Nonparallelism of the two eyes indicates muscle imbalance because of weakness or paralysis of the deviant eye. In a second method, the child is asked to follow the movement of a small object (e.g., a pencil point, lighted penlight) as it is moved through the extremes of what are called the six cardinal positions of gaze. In extreme lateral gaze, normal subjects can show a few quick beats of a jerky or nystagmoid movement. Nystagmoid movement is abnormal if it is prolonged or present in any other eye posture. The third method, called the cover–uncover test, eliminates binocular fusion as a factor in maintaining parallelism between the eyes and is used to determine which eye is used for fixation. The child’s attention is directed toward a fixed object such as a small picture or tongue blade. A light should not be used because it may not stimulate accommodation. If a mild weakness is present, the eye with blocked vision drifts into a resting position, the extent of which depends on the relative strength of the muscles. The eye should snap back when the card is removed. This test is always done for near and far fixation. Visual acuity is evaluated to obtain a comparison of the two eyes. A tumbling E chart (or similar test chart) can be used for young children.46 Treatment of strabismus is directed toward the development of normal visual acuity, correction of the deviation, and superimposition of the retinal images to provide binocular vision. Nonsurgical and surgical methods can be used. In children, early treatment is important; the ideal age to begin is 6 months. Nonsurgical treatment includes occlusive patching, pleoptics (i.e., eye exercises), and prism glasses. Because prolonged occlusive patching leads to loss of useful vision in the covered eye, patching is alternated between the affected and unaffected eye. This improves the vision in the affected eye without sacrificing vision in the unaffected eye. Prism glasses compensate for an abnormal alignment of an optic globe. Occasionally, longacting miotics in weak strengths (e.g., echothiophate iodide solution [Phospholine Iodide] or pilocarpine [Pilocarpine HS]) are used to cause pharmacologic accommodation in place of or in combination with corrective lenses.41 Surgi- AMBLYOPIA 1326 UNIT XIII Special Sensory Function acuity improves slowly, requiring several months to reach normal levels. red, swollen, and watering. She takes him to the pediatrician in the morning and is told that he has “pink eye.” She is told that the infection should go away by itself. In summary, binocular vision depends on three pairs of A. What part of the eye is involved? extraocular muscles–the medial and lateral recti, which move the eye from side to side; the superior and inferior recti, which move the eye up and down; and the superior and inferior obliques, which rotate the eye around its optical axis. The extraocular muscles are innervated by three pairs of cranial nerves: (1) the trochlear nerve (CN IV), which innervates the superior oblique and turns the eye downward and laterally; (2) the abducens nerve (CN VI), which innervates the lateral rectus and moves the eye laterally; and (3) the oculomotor nerve (CN III), which innervates the medial rectus and turns the eye medially, the superior rectus, which elevates the eye and rolls it upward, the inferior rectus, which depresses the eye and rolls it downward, and the inferior oblique, which elevates the eye and turns it laterally. For full visual function, it is necessary that the two eyes point toward the same fixation point and the two images become fused. Binocular fusion is controlled by ocular reflex mechanisms that adjust the orientation of each eye to produce a single image. The term conjugate gaze refers to the use of both eyes to look steadily in one direction. During conjugate eye movements, the optical axes of the two eyes are maintained parallel with each other as the eyes rotate upward, downward, or from side to side in their sockets. Smooth pursuit movements are tracking movements that serve to maintain an object at a fixed point in the center of the visual fields of both eyes. Saccadic eye movement consists of small jumping movements that represent rapid shifts in conjugate gaze orientation. Vergence movements (convergence and divergence) are necessary for maintaining the focus on a near image. Strabismus refers to abnormalities in the coordination of eye movements with loss of binocular eye alignment. This inability to focus a visual image on corresponding parts of the two retinas results in diplopia. Esotropia refers to medial deviation, exotropia refers to lateral deviation, hypertropia refers to upward deviation, hypotropia refers to downward deviation, and cyclotropia refers to torsional deviation. Paralytic strabismus is caused by weakness or paralysis of the extraocular muscles. Nonparalytic strabismus results from the inappropriate length or insertion of the extraocular muscles or from accommodation disorders. Amblyopia (i.e., lazy eye) is a condition of diminished vision that cannot be corrected by lenses and in which no detectable organic lesion in the eye can be observed. It results from inadequately developed CNS circuitry because of visual deprivation (e.g., cataracts) or abnormal binocular interactions (e.g., strabismus, anisometropia) during visual immaturity. B. What type of conjunctivitis do you think this child has: bacterial, viral, or allergic? REVIEW EXERCISES The mother of a 3-year-old boy notices that his left eye is red and watering when she picks him up from day care. He keeps rubbing his eye as though it itches. The next morning, however, she notices that both eyes are C. Why didn’t the pediatrician order an antibiotic? D. Is the condition contagious? What measures should the mother take to prevent its spread? During a routine eye exam to get new glasses because she had been having difficulty with her distant vision, a 75-year-old woman is told that she is developing cataracts. A. What type of visual changes occur as the result of a cataract? B. What can the woman do to prevent the cataracts from getting worse? C. What treatment may she eventually need? A 50-year-old woman is told by her eye doctor that her intraocular pressure is slightly elevated and that although there is no evidence of damage to her eyes at this time, she is at risk for developing glaucoma and should have regular eye exams. A. Describe the physiologic mechanisms involved in the regulation of intraocular pressure. B. What are the risk factors for development of glaucoma? C. Explain how an increase in intraocular pressure produces its damaging effects. The parents of a newborn infant have been told that their son has congenital cataracts in both eyes and will require cataract surgery so that he does not lose his sight. A. Explain why the infant is at risk for losing his sight if the cataracts are not removed. B. When should this procedure be done to prevent loss of vision? References 1. Leonard R. (1999). Statistics on visual impairment: A resource manual. Lighthouse International. [On-line]. Available: http:// www.lighthouse.org. 2. Sullivan J.H., Shetlar D.J., Whitcher J.P. (2004). Lids, lacrimal apparatus & tears. In Riordan-Eva P. Vaughan & Ashbury’s general ophthalmology (16th ed., pp. 80–99). New York: Lange Medical Books/McGraw-Hill. 3. Morrow G.L., Abbott R.L. (1998). Conjunctivitis. American Family Physician 57(4), 735–746. 4. Leibowitz H.M. (2000). The red eye. New England Journal of Medicine 343(5), 345–351. 5. Riordan-Eva P. (2003). Eye. In Tierney L.M., McPhee S.J., Papadakis M.A. (Eds.), Current medical diagnosis and treatment (42nd ed., pp. 146–177). New York: Lange Medical Books/ McGraw-Hill. CHAPTER 54 6. Collum L.M.T., Kilmartin D.J. (2001). Acute allergic conjunctivitis. In Abelson MB. Allergic diseases of the eye (pp. 112–131). Philadelphia: W.B. Saunders. 7. Klintworth G.K. (1999). The eye. In Rubin E., Farber J.L. (Eds.), Pathology (3rd ed., pp. 1537–1543). Philadelphia: LippincottRaven. 8. Olitsky S.E., Nelson L. (2004). Disorders of the eye. In Behrman R.E., Kliegman R.M., Jenson H.B. (Eds.), Nelson textbook of pediatrics (17th ed., pp. 2099–2102, 2105–2111). Philadelphia: W.B. Saunders. 9. Biswell R. (2004). Cornea. In Riordan-Eva P. Vaughan & Ashbury’s general ophthalmology (16th ed., pp. 129–153). New York: Lange Medical Books/McGraw-Hill. 10. Shaikh S., Ta C. (2002). Evaluation and management of herpes zoster ophthalmicus. American Family Physician 66, 1723–1732. 11. Kandel E.R., Schwartz J.H., Jessell T.M. (2000). Principles of neural science (4th ed., pp. 523–547). New York: McGraw-Hill. 12. Riordan-Eva P. (2004). Glaucoma. In Riordan-Eva P. Vaughan & Ashbury’s general ophthalmology (16th ed., pp. 212–229). New York: Lange Medical Books/McGraw-Hill. 13. Guyton A.C., Hall J.E. (2000). Textbook of medical physiology (10th ed., pp. 575–576). Philadelphia: W.B. Saunders. 14. Martin X.D. (1992). Normal intraocular pressure in man. Ophthalmologica 205, 57–63. 15. Coleman A.L. (1999). Glaucoma. Lancet 354(9192), 1803–1810. 16. Distellhorst J.S., Hughes G.M. (2003). Open-angle glaucoma. American Family Physician 67, 1937–1950. 17. Alward W.L.M. (1998). Medical management of glaucoma. New England Journal of Medicine 339, 1299–1307. 18. Kipp M.A. (2003). Childhood glaucoma. Pediatric Clinics of North America 50, 89–104. 19. Quillen D.A. (1999). Common causes of vision loss in elderly patients. American Family Physician 60, 99–108. 20. Harper R.A., Shock J.P. (2004). Lens. In Riordan-Eva P. Vaughan & Ashbury’s general ophthalmology (16th ed., pp. 212–229). New York: Lange Medical Books/McGraw-Hill. 21. Schmitt C., Hockwin O. (1990). The mechanisms of cataract formation. Journal of Inherited Metabolic Disease 13, 501–508. 22. Chylack L.T. (1997). Cataracts and inhaled corticosteroids. New England Journal of Medicine 337(1), 44–48. 23. Jobling A.J., Augusteyn R.C. (2002). What causes steroid cataracts? A review of steroid-induced posterior subcapsular cataracts. Clinical and Experimental Optometry 852, 61–75. 24. Solomon B., Donnenfeld E.D. (2003). Recent advances and future frontiers in treating Age-related cataracts. JAMA 290(2), 248–251. 25. Phelan J.K. (2000). A brief review of retinitis pigmentosa and the identified retinitis pigmentosa genes. Molecular Vision 6, 116–124. 26. Albert D.M., Dryja T.P. (1999). The eye. In Cotran R.S., Kumar V., Collins T. (Eds.), Robbins pathologic basis of disease (6th ed., pp. 1359–1377). Philadelphia: W.B. Saunders. 27. Poole T.R.G., Graham E.M. (2004). Ocular disorders associated with systemic disease. In Riordan-Eva P. Vaughan & 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. 41. 42. 43. 44. 45. 46. 47. 48. Disorders of Visual Function 1327 Ashbury’s general ophthalmology (16th ed., pp. 307–342). New York: Lange Medical Books/McGraw-Hill. Fong D.S., Aiello L., Gardner T.W., et al. (2003). Diabetic retinopathy. Diabetes Care 26(1), 226–229. Cuilla T.A., Amador A.G., Zinman B. (2003). Diabetic retinopathy and diabetic macular edema. Diabetes Care 26, 2653–2664. Donaldson M., Dockson P. (2003). Medical treatment of diabetic neuropathy. Eye 17, 550–562. Hardy R.A., Shetlar D.J. (2004). Retina. In Riordan-Eva P. Vaughan & Ashbury’s general ophthalmology (16th ed., pp. 189–211). New York: Lange Medical Books/McGraw-Hill. Ferris F.L., Davis M.D., Aiello L.M. (1999). Treatment of diabetic retinopathy. New England Journal of Medicine 341, 667–678. Diabetes Control and Complications Trial Research Group. (1993). The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New England Journal of Medicine 329, 977–986. Adler A.I., Stratton I.M., Neil A.W., et al. (2000). Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): Prospective observational study. British Medical Journal 321, 412–419. Fine S.L., Berger J.W., MacGuire M., Ho A.C. (2000). Age-related macular degeneration. New England Journal of Medicine 342, 483–492. Chopdar A., Chakravarthy U., Verma D. (2003). Age-related macular degeneration. British Medical Journal 326, 484–488. Gottlieb J.L. (2002). Age-related macular degeneration. JAMA 288(18), 2233–2236. Lewis R.A., Lupski J.R. (2000). Macular degeneration: The emerging genetics. Hospital Practice 35(6), 41–58. Jampol L.M., Ferris F.L. (2001). Antioxidants and zinc to prevent progression of age-related macular degeneration. JAMA 286(19), 2466–2468. Fredrick D.P., Asbury T. (2004). Strabismus. In Riordan-Eva P. Vaughan & Ashbury’s general ophthalmology (16th ed., pp. 230–249). New York: Lange Medical Books/McGraw-Hill. Mills M.D. (1999). The eye in childhood. American Family Physician 60, 907–918. Lavrich J.B., Nelson L.B. (1993). Diagnosis and management of strabismus disorders. Pediatric Clinics of North America 40, 737–751. Ticho B.H. (2003). Strabismus. Pediatric Clinics of North America 50, 173–188. Kline L.B., Bajandas F.J. (1996). Neuro-ophthalmology review manual (Chapters 4–7). Thorofare, NJ: Slack. Broderick P. (1998). Pediatric vision screening for the family physician. American Family Practitioner 58(3), 691–704. Scheiman M. (1997). Understanding and managing vision defects (pp. 26–27). Thorofare, NJ: Slack. Mittelman D. (2003). Amblyopia. Pediatric Clinics of North America 50, 189–196. Simon J.W., Kaw P. (2001). Commonly missed diagnoses in childhood eye exams. American Family Physician 64, 623–628.