* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 4_part 1
Stoichiometry wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Biochemistry wikipedia , lookup
Bent's rule wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Chemical reaction wikipedia , lookup
Bond valence method wikipedia , lookup
Chemical bond wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Marcus theory wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Click chemistry wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Rate equation wikipedia , lookup
Hydroformylation wikipedia , lookup
George S. Hammond wikipedia , lookup
Transition state theory wikipedia , lookup
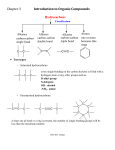
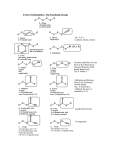
Chapter 4 & 5 Alkenes and Alkynes *Skip section 4.4, 5.5, 5.7 I. Molecular formula (3.3, 4.1) Alkane CnH2n+2 = a saturated hydrocarbon E.g C-C-C-C-C-C E.g C H or C H add a ring: hexane add π bond: *2 missing H’s = a site/degree of unsaturation (DU) Determine degree of unsaturated (DU) from the molecular formula and structural formula One degree of unsaturation is equivalent to 1 ring or 1 double bond (1 π bond). Two degrees of unsaturation is equivalent to 2 double bonds, 1 ring and 1 double bond, 2 rings, or 1 triple bond (2 π bonds). E.g C9H16 II. Determine formula Alkene structure (4.1) CHM 201-Dang1 3-D sketch 3-D sketch Cis-trans isomerism • • Because of restricted rotation about a carbon-carbon double bond, an alkene with two different groups on each carbon of the double bond shows cis-trans isomerism. Aka as geometric isomers Nomenclature for Alkenes and Alkynes (4.2, 4.5, 5.7) To name an alkene; The parent name is that of the longest chain that contains the C=C. Number the chain from the end that gives the lower numbers to the carbons of the C=C. Locate the C=C by the number of its first carbon. Use the ending -ene to show the presence of the C=C Branched-chain alkenes are named in a manner similar to alkanes in which substituted groups are located and named Don’t forget to include the configuration where stereochemistry is appropriate (cis, trans, E, Z) A compound with two double bonds is called –diene (or triene, tetraen, etc..) If the structure contains a triple bond, it is named as an “#-alkyne.” CHM 201-Dang2 CHM 201-Dang3 *Greater atomic number => higher priority If two groups are the same consider the next atom and so on … Alkene and Alkyne Physical Properties (B&P 4.3) Nonpolar molecules have same trends in boiling points and solubility “like dissolves like” Which has a higher boiling point? 1-hexene or 1-pentyne Alkene stability (B&P 5.6) and conjugated dienes **If more than one π bonds present, then conjugated π systems are more stable than conjugated CHM 201-Dang4 More alkyl substituents bonded to the sp2 carbon of alkene, the greater its stability E.g Which is more stable ? What can we investigate from a chemical reaction? A. Thermodynamics i. Compare E (stability) of starting materials (SM) and products ΔH < 0, ΔG <0 ii. Predict direction of equilibrium (forward or reverse) ΔG = ΔH - TΔS CHM 201-Dang5 *Energy vs. Progress of Rxn Diagrams ΔH >0, ΔG >0 E is consumed Reactants favored Reactants lower E, more stable Reverse rxn is spontaneous ΔH < 0, ΔG <0 Products are favored Products lower E, more stable Forward rxn is spontaneous Kinetics: -rate of reaction A + B C + D Rate = k [A]x [B]y k = rate constant @ given temperature E.g CH3—Cl + -OH CH3—OH + Cl- If [-OH] is doubled, rate doubles If [CH3Cl] is doubled, rate doubles Rate law = k [-OH][CH3Cl] CHM 201-Dang6 The rate is “first order” with respect to each reactant (exponent = 1) and “second-order” overall (sum of exponents) Reaction Rate Variable (4.9) -to achieve TS, need a collision with enough Energy 1. Increasing temperature, increasing rate 2. Increasing concentration, increasing rate 3. decreasing activation Energy (Ea), increasing rate Rate Determining Step -Step with highest T.S Energy - has highest Ea + is slowest Reactive Intermediates - High energy species (unstable) - Produced then consumed in reaction (doesn’t appear in net rxn) - Different from transition state (T.S) Energy vs. Progress of reaction (Reaction Coordinate) (4.9) Diagram for Two-Step Mechanism CHM 201-Dang7 E.g CHM 201-Dang8