* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Don`t forget to study the generic functional groups and the common
Survey
Document related concepts
Woodward–Hoffmann rules wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Elias James Corey wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Stille reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Asymmetric induction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
George S. Hammond wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Kinetic resolution wikipedia , lookup
Petasis reaction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Hydroformylation wikipedia , lookup
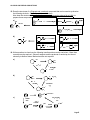
Transcript
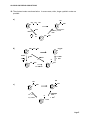
ALCOHOLS & PHENOLS SOLUTIONS Don’t forget to study the generic functional groups and the common alkyl groups (isobutyl, etc). 1. IUPAC = I and common = c. a) d) 4-isopropyl-2-methylphenol 6-methyl-4-propyl-3-heptanol (this is the longest chain containing the -C and with the most branch points) e) b) phenylmethanol (I) benzyl alcohol (c) 4-cyclopentyl-2,2-butanediol f) 3-penten-2-ol c) (Z)-4-methyl-2-cyclohexen-1-ol or cis-4-methyl-2-cyclohexen-1-ol g) 2-cyclopenten-1-ol 2. Draw structures of the following compounds: a) glycerol OH OH OH d) hydroquinone OH CH2 CH CH2 (also glycerin or 1,2,3-propanetriol) OH e) -naphthol b) m-cresol OH OH CH3 c) resorcinol f) allyl alcohol OH OH CH2CH CH2 OH Page 1 ALCOHOLS & PHENOLS SOLUTIONS 3. Give IUPAC names and, where possible, common names for the following compounds. a) OH d) CH3 CH2 HO 3 4 CH CH2 CH CH3 1 2 CH3 (c) sec-butyl alcohol (I) 2-butanol CH3 3-(1-ethyl-3-methylbutyl)phenol e) b) H3C C OH CH3 Br OH OH (c) t-butyl alcohol (c) 2-methyl-2-propanol CH2CHCH2CH2CHCH3 5-bromo-1,2-hexanediol f) OH OH c) OH isobutyl alcohol (c) 2-methyl-1-propanol (I) catechol (c) 1,2-dihydroxybenzene (I) 2-hydroxyphenol (I) 4. Draw structures of the following compounds a) isoamyl alcohol d) 2-ethyl-2-buten-1-ol OH HO e) trans-2-ethyl-3-methyl-2-penten-1-ol b) 1-cyclopentyl-1-phenyl-2-propanol OH HO c) cis-2-iodocyclohexanol f) (E)-1-chloro-1,2-cyclobutanediol OH Cl I OH H OH Page 2 ALCOHOLS & PHENOLS SOLUTIONS 5. Note that this is a 3° alcohol. a) Br HBr, H2O SN1 OH H2O + CH3 CH3 1-bromo-1-methylcyclohexane b) O- Na+ Na Na metal is a very strong base. It deprotonates alcohols. 1/2 H2 + CH3 sodium 1-methylcyclohexoxide c) H2SO4 CH3 3° alcohols are readily dehydrated by sulfuric or phosphoric acids and moderate heating H2O + 1-methylcyclopentene d) cold Na2CrO4, H2SO4, H2O e) PBr3 NO REACTION 3° are only oxidized under severe (hot) conditions with strong oxidants Br + CH3 This is the same product as obtained with HBr Again the mechanism is SN1 for 3° alcohols PBr2OH 6. What Grignard and what carbonyl will react to produce the following. Draw structures of all possible combinations. O O C CH3 + CH3MgBr OR + 2 CH3MgBr H3C OH C(CH3)2 C CH3 MgBr + O C 7. What hydride and what carbonyl will react to produce the following. Draw structures of all possible combinations. CH3 CH3 OH C CH2 CH3 CH3 O C C H CH3 CH3 CH3 + NaBH4 CH3 O C C OR CH3 + CH3 2 LiAlH4 CH3 O C C OH CH3 + 3 LiAlH4 Page 3 ALCOHOLS & PHENOLS SOLUTIONS 8. List the reagents needed for the following transformations a) CH3 CH2 CHBr CH3 CH3 CH CH c) CH CH CH3 CH3 CH2 CH3 CH(OH) CH2 CH3 CH(OH) CH3 CH3 CH2 KOH H3PO4 + H2O or H2SO4 + H2O b) CH3 CH3 CHBr CH3 Any strong base will cause this 2° alkyl halide to dehydrohalogenate via E2 1 Hg(CH3COO-)2, THF, H2O or 2 NaBH4 Any of the standard reagents used for hydration of alkenes HBr or PBr3 Either reagent causes 2° alcohols to undergo SN2. O d) CH3 CH2 CH(OH) CH3 CH3 CH2 C PCC or Collins CH3 or KMnO4 or HNO3 Any mild or moderate oxidant will oxidize a 2° alcohol to a ketone e) CH3 CH2 CH(OH) f) g) CH3 CH3 CH2 CHCl CH3 HCl or SOCl2 will convert alcohols to alkyl chlorides Only a rhodium catalyst allows hydrogenation of H2/Rh an aromatic ring at standard pressure and temperature. Carboxylic acids are not reduced by hydrogen. COOH COOH CH2OH COOH Only this reagent will reduce 1 LiAlH4 carboxylic acids but it does not 2 H2O reduce alkene/alkyne unsaturation 9. Number the following compounds in order of acidity, i.e., 1 = most acidic, 4 = least acidic OH OH COOH NO2 3 4 1 2 Carboxylic acids are more acidic than phenols. The nitro group is electron withdrawing so it will help stabilize the negative phenoxide ion after deprotonation, making it more acidic than phenol. Benzene has only non polar C-H bonds, they are not acidic. Page 4 ALCOHOLS & PHENOLS SOLUTIONS 10. The shortest routes are shown below. In some cases, other, longer synthetic routes are possible. a) OH CH2 CH2 OH CH H2O Markovnikov H2SO4 product H2O POCl3, N b) CH2 POCl3, CH E2 CH2 CH2 OH H2O CH2OH 1 LiAlH4 2 H2O KMnO4 N O or CH2 CH CH3 C OH hot KMnO4 OH OH c) CH C CH3 CH3 CH3 Cr+6, H+ any oxidant 1 CH3MgBr ether O C + 2 H3O CH3 Page 5 ALCOHOLS & PHENOLS SOLUTIONS 11. This one is tougher. Note the addition of the ethyl group. The only methods we’ve learned for adding Carbon groups are 1) chain lengthening terminal alkynes, 2) FC alkylations/acylations to aromatics, 3) Grignards, organosodium and Gilman reactions. Obviously the first two don’t apply here, so look for an opportunity to perform a Grignard rxn, i.e., synthesize a carbonyl to add the Grignard to: OH OH CH2CH3 oxidize Cr+6, H+ 1 BH3, THF 2 NaOH, H2O2, pH8 1 CH3CH2MgBr, ether + 2 H3O O antiMarkovnikov addition conc. H2SO4 CH2CH3 3° Grignard reaction dehydration CH2CH3 OH 12. For the following reaction: a) Write products for the reaction b) Show the mechanism of the reaction .. O .. c) Calculate pKeq .. O: .. H + Na OH - Na+ + H2O pKb = -1.74 phenol pKa = 9.9 sodium phenoxide pKeq = pKa + pKb - 14 = (9.9 -1.74 - 14) = -5.8 (100% extent of reaction) 13. This 3° alcohol is undergoing an SN1 reaction. OH is a poor leaving group, but once protonated by a strong acid, a better leaving group is formed (H2O). CH3 H3C C .. O .. H H .. Cl : .. 3° CH3 SN1 CH3 H3C C H + O .. H3C C Cl CH3 H H3C H2O C+ .. : .. Cl : Page 6 ALCOHOLS & PHENOLS SOLUTIONS 14. Remember to consider whether the substrate is 1°, 2°, or 3° CH3 CH3 a) KOH Br b) H3C CH2 CH OH + C PCC is a mild oxidant, oxidizing 1° alcohols to aldehydes H H3C H3C c) This 3° halide undergoes E2 rxn. with strong bases H2O O H3C PCC in CH2Cl2 CH KBr + O OH Jones Reagent CH 2° alcohols are oxidized to ketones by mild or moderately strong oxidants CH3 C CH3 O d) 1. Cl CH3 H3C S CH3 O C CH2 C H3C OH O CH3 CH3 LiAlH4 then H3O+ 2. HO S CH3 O H H + Tosyl chloride converts the OH group to a good leaving group, which is then displaced by Hydride in an SN2 reaction. e) Na OH 2° f) O- Na+ + Br + PBr3 OH POCl3 CH3CH2CH2CH2OH g) CH3CH2CH CH2 PBr2OH H3C SOCl2 CH CH2 and pyridine acts as a strong bulky base forcing an E2 dehydration reaction even in 1° alcohols. H3C i) H3C CH OH HCl CH C CH2Cl SO2 H3C O OH O CH3 1 LiAlH4 2 H2O CH2 CH Like HBr, this reagent converts alcohols to alkyl bromides. The mechanism is SN2 POCl3 converts to OH group to a better leaving group + H2O N: h) CH2 Very strong bases like Na metal 1/2 H2 deprotonate weakly acidic protons in water and alchols CH2 Thionyl chloride coverts all alcohols to alky halides. The OH group is converted to a good leaving group. This 1° alcohol reacts via an SN2 reaction LiAlH4 reduces carboxylic acids and esters to 1° alcohols but it does not reduce C to C unsaturation. Page 7 ALCOHOLS & PHENOLS SOLUTIONS 15. Draw the structures of a Grignard and a carbonyl compound that can be used to synthesize the following alcohols. Show all possible combinations. Also draw the structures of any carbonyl compounds that can be reduced by a LiAlH4 to prepare these compounds O a) O OH C MgBr C 2 CH3CH2MgBr + OR CH3CH2 C CH2CH3 + CH2CH3 O CH2CH3 C CH2CH3 O b) OH C + CH3CH2MgBr O + OR C 2 LiAlH4 C OH + 3 LiAlH4 H H O O + H C NaBH4 C H H + MgBr 16. Write equations to show how the following transformations can be carried out. More than one step may be required. Show all reagents and the products of each step, but do not show any transition states. Mechanisms are not required a) OH OH CH3 Cr+6, H+ 1 CH3MgBr O b) + 2 H3O OH O CH3 CH3 Na CH2CH3 or CH3CH2Cl Na+NH2O- Na+ SN2 CH3 O c) CH2CH2CH3 CH3CH2CH2I H2SO4 SO3 300° C 1 NaOH SO3H + 2 H3O SN2 OH NaOH O- Na+ acid/base Page 8 ALCOHOLS & PHENOLS SOLUTIONS :O.. 17. . C(CH3)3 H+HSO4- H CH Zaitsev product (CH3)2C CH3 C(CH3)2 E1 CH3 + H :O C(CH3)3 CH C H3C H C CH3 CH3 H CH3 CH3 H3C C + C CH3 H HSO4- 3° CH3 rearrangement via methide shift 2° 18. Draw the structure(s) of all reagents which would give the following alcohol when reduced with LiAlH4 (CH3)2CHCH2OH (CH3)2CHC O O O H (CH3)2CHC or (CH3)2CHC or OR OH 19. Draw the structures of all sets of reagents that would give the following alcohol when reduced with a Grignard reagent. Show Grignards as well as other reagents. O OH CH3CH2CCH2CH3 CH3CH2CCH2CH3 + CH3 O O CH3CH2CCH3 or 2 CH3CH2MgBr CH3CH2MgBr + CH3MgBr + CH3COR or 20. Draw the formulas or structures of the products of the following reactions ... a) H3C H3C 2° H3C POCl3 POCl3 converts the OH group to a good leaving group. Pyridine acts as a strong bulky base causing E2 dehydration to the alkene. Two products form. + OH N b) H3C H3C 2° PBr3 Br OH PBr2OH + PBr3 works like HBr to convert alcohols to halides. SN2 H3C c) d) H3C cold KMnO4 aqueous 2° O OH H3C hot concentrated HNO3 2° alcohols are normally oxidized to ketone except under severe conditions in which case they are oxized to carboxylic acids and cleaved. O H3C O C OH C Cleavage occurs on either side of the -carbon H3C OH OH OH C + C OH O O Page 9 ALCOHOLS & PHENOLS SOLUTIONS H3C 21. 3-methylcyclohexanol potassium 3-methylcyclohexoxide H3C K O- K+ OH + 1/2 H2 K (like Na and Li) is a very strong base and deprotonates the weakly acidic alcohol in an acid/base reaction. This is a rapid lab test for identifying alcohols; H2 gas is evolved. 22. Remember to think backwards from the product as well as forwards from the reagent. a) CH2CH2OH CH2OH KMnO4 H3O+ 1 LiAlH4 2 H2O O COH b) Br 1° OH CH2 CH3 H2SO4 H2O KOH SN2 Markovnikov product OH POCl3, CH2 CH2 E2 N 1° OH c) SO3 H2SO4 CH2Br (PhCO2)2 NBS SO3H OH 1 NaOH, 300°C + 2 H3O OH AlCl3 CH3 CH3Cl d) H3C O H3C CH C C OCH3 C H3C H3C 2 equiv. 1 C6H5MgBr + 2 H3O OH H3C CH C POCl3, N or H2SO4 H3C Page 10