* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Carboxylates/esters vs ketones/aldehydes
Survey
Document related concepts
Kinetic resolution wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Aromaticity wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Discodermolide wikipedia , lookup
George S. Hammond wikipedia , lookup
Bottromycin wikipedia , lookup
Aromatization wikipedia , lookup
Elias James Corey wikipedia , lookup
Aldol reaction wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Stille reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Asymmetric induction wikipedia , lookup
Marcus theory wikipedia , lookup
Hydroformylation wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Transcript
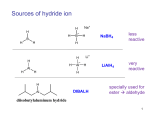
Chem 216 S11 Notes - Dr. Masato Koreeda Date: May 10, 2011 Topic: _Experiment 4__ page 1 of 3. Experiment 4: NaBH4 Reduction of an Unknown Aromatic Ketone [See: Ege’s book, Section 14.4, pp 545-549] Two commonly used hydride-based reducing agents: 1. Sodium borohydride (NaBH4) H Na+ H B- H H electronegativity values: H 2.1; B 2.0; Al 1.5 |Δe.n.| for B-H: 0.1 |Δe.n.| for Al-H: 0.6 - mild reducing agent - relatively stable reagent (against moisture, air) 2. Lithium aluminum hydride (LiAlH4) H a stronger H donor H Al- H more polarized, more on the H H In addition, the size differences between B and H and Al and H should make the dissociation of H- more effective for the Al-H bond. - powerful reducing agent - reacts violently with water, ROH to produce H2 gas - Reactions with LiAhH are usually carried out in a polar aprotic solvent such as anhydrous tatrahydrofuran (THF) and anhydrous (diethyl) ether (CH3CH2OCH2CH3) Li+ O O THF (diethyl) ether In addition, the difference in the coordination power of Na+ and Li+ (stronger) on the carbonyl oxygen further contributes to make the reactivity of LiAlH4 stronger. Reduction with LiAlH4 requires an aqueous (usually acidic) workup. Carboxylates/esters vs ketones/aldehydes ease of reduction C=O C more electrophilic R C O O R C O R' O R C R' O carboxylate ester ketone (carboxylic R C H O aldehyde acid)* NaBH4 reduces LiAlH4 reduces * R C O H O Li H H Al H H H R C O Al H O H Li + H2 H3O+-workup RCH2OH Chem 216 S11 Notes - Dr. Masato Koreeda Topic: _Experiment 4__ page 2 of 3. Date: May 10, 2011 NaBH4 reduces ketone and aldehyde carbonyls to their corresponding alcohols and reduces ester/lactone carbonyls extremely slowly at room temperatures Therefore, selective reduction of a ketone/aldehyde in the presence of an ester/lactone group in the same molecule is generally attainable. For example, with NaBH4: O OH NaBH4 H3CO H3COH room temp. O OH + H3CO H3CO cis-isomer O O trans-isomer In contrast, with LiAlH4: 1. LiAlH4/ether 0 °C O OH 2. H3O+ -workup H3CO OH + HO O HO cis-isomer trans-isomer An intramolecular reaction involving a ketone/aldehyde C=O and an ester C=O during NaBH4 reduction reaction is often observed: O OCH3 NaBH4 O C2H5OH room temp. O OH O + O O + OCH3 O NaBH4 -O O reacts faster with the nearby ester C=O than protonation by the solvent O OCH3 O OCH3 intramol. reaction O O + OCH3 O + OCH3 O cis O OCH3 O O O OCH3 O trans attack to the ester C=O C These reaction rates are comparable. protonation from the solvent C2H5OH OH OCH3 O Chem 216 S11 Notes - Dr. Masato Koreeda Topic: _Experiment 4__ page 3 of 3. Date: May 11, 2011 Mechanism of NaBH4 reduction [see: Ege’s, p. 546]. (solvent) H OC2H5 δO δ+ Na H H B H H in C2H5OH O H OH Na H + BH3 + Na OC2H5 BH3 becomes B(OC2H5)3 by reacting with ethanol, then, when heated with water, becomes B(OH)3. The mechanism of the NaBH4 reduction in a protic solvent such as ethanol, methanol, and water is known to be quite complex since NaBH4 reacts with the solvent, e.g., NaBH4 + C2H5OH → NaBH3(OC2H5) + H2 Because of this, usually at least one mol. equivalents of NaBH4 are used for the reaction in a protic solvent. What makes the kinetics of the reaction more complicated is the fact that these solvent reacted reagents such as NaBH3(OC2H5) and NaBH2(OC2H5)2 reduce ketones/aldehydes much faster than the original NaBH4 does. Pre-lab Experiments: In Experiment 4, you need to go through a few pre-lab experiments (see p. 10 of the lab manual) on your unknown aromatic ketone. After narrowing down to one or two possibilities for the structure of your unknown aromatic ketone, obtain the information on the mp/bp and IR of your expected REDUCTION product(s) (i.e., an alcohol, not your starting ketone) through the use of Reaxys [https://www.reaxys.com/reaxys/secured/start.do;jsessionid=8DEDFF0EA14EB7ABC856F12631DBD 642]. The web address of the URL user guide of the Reaxys written by Dr. Ye Li is: http://guides.lib.umich.edu/chem216 If your unknown ketone is likely to be O Draw the structure of its reduction product on the “structure editor” screen of the Reaxys program. OH The information you need to obtain is: bp or mp (the solvent used for recrystallization) and where to find the IR data or spectrum of the reduction product. Please note that the Reaxys program does not show you any IR data/spectra. It only gives you references to find such information.