* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 06a Organic Acids 2
Survey
Document related concepts
Nucleic acid analogue wikipedia , lookup
Genetic code wikipedia , lookup
Catalytic triad wikipedia , lookup
Microbial metabolism wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Peptide synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
15-Hydroxyeicosatetraenoic acid wikipedia , lookup
Butyric acid wikipedia , lookup
Biosynthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Transcript
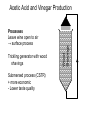
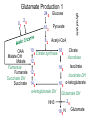
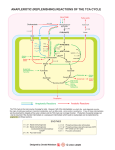
Acetic Acid and Vinegar Production History • As old as wine making (10,002 y) • Hannibal Uses: • Food acid and preservative, • medical agent • Volatile (not for cooking) Biochemistry Aerobic incomplete oxidation of organics to acetic acid TCA cycle not fully operating Substrates: Ethanol, glucose, hydrocarbons Acetic Acid and Vinegar Production -4 0 12 2 20 10 2 20 82 ETP 6 ATP 00 12 2 = CH3-CH2OH 2 0 = 2 red. equiv. 10 2 = CH3-CH2O -4 0 = O2 8 2 = CH3-COOH Bacteria Underoxidiser: Gluconobacter Overoxidiser: Acetobacter (can totally oxidise to CO2) Processes Leave wine open to air → surface process Trickling generator with wood shavings Submersed process (CSTR) + more economic - Lower taste quality Wood Shavings Acetic Acid and Vinegar Production Acetic Acid and Vinegar Production Downstream Only filtering to remove biomass Critical process conditions: • 30°C (Cooling required for CSTR) • Maximum ETOH concentration: 13% 50% inactive cells after 1 min air off due to acetaldehyde accumulation ↑ [etOH] + ↑ [acetic acid] + ↓ [O2] → ↑ acetaldehyde Product yield (g ac./ g etOH): up to 98% Citric Acid Production Special properties: Complexing agent for metals (Fe, Ca) Uses: • Principle food acid in soft drinks, jams • Food preservative • Medical: iron citrate as iron supplement anticoagulant for storage of blood • Detergent to replace phosphorus thus avoiding eutrophication • Used in metal cleaning fluid • Used as siderphore by microbes Fe(OH)3 + citrate → Fe3+ - citrate complex (not available for uptake by cells) → bio-available Citric Acid Production Biochemistry TCA cycle, Glyoxylate cycle Gaden’s fermentation type II • Trophophase: growth and complete substrate oxidation to CO2 • Idiophase: deregulated TCA cycle due to iron limitation: ↓↓α-ketoglutarate DH, ↓ Aconitase ↓ Isocytrate lyase, ↑ Citrate synthase. Why? Citric Acid Production Reasons for citrate excretion: 1. Aconitase contains an iron sulfur centre Thus Fe limitation → citrate conversion inhibited 2. Citrate is a siderophore Thus iron limitation can be expected to stimulate citrate synthase Problem: Citrate excretion → interruption of TCA cycle → no more OAA, citrate excretion ceases Solution: Pyruvate carboxylase (key enzyme for citric acid production): Pyruvate + CO2 → OAA 10 3 + 0 1 → 10 4 Anaplerotic sequences to replenish reactions of TCA cycle (usually for biosynthesis) TCA Cycle – Electron and Carbon Flow Citric acid synthesis during trophophase 10 4 12 4 12 4 14 4 Glucose 10 3 Pyruvate 82 Acetyl-CoA Citrate synthase 18 6 glycolysis OAA Malate DH Malate Fumarase Fumarate Succinate DH Succinate 24 6 Citrate Aconitase 18 6 Isocitrate 16 5 Isocitrate DH α-ketoglutarate α-ketoglutarate DH How can the cycle continue when citrate is excreted? TCA Cycle – Metabolites 8 2 Acetyl-CoA CH2-COOH Citrate COH-COOH 18 6 CH2-COOH α-ketoglutarate HOOC-CH2-CH2-CO-COOH 1-6-6-2-1 10 4 OAA HOOC-CO-CH2-COOH 16 5 12 4 Fumarate HOOC-CH=CH-COOH 1-5-5-1 14 4 Succinate HOOC-CH2-CH2-COOH 1-6-6-1 12 4 Malate HOOC-CH2-CHOH-COOH 1-6-4-1 10 3 Pyruvate CH3-CO-COOH How can the cycle continue when citrate is excreted? TCA Cycle – Citrate isomerisation Citrate CH2 - COOH | HOCOH -COOH | CH2 - COOH CH2 - COOH | cis-Aconitate CH - COOH || HOCH - COOH CH2 - COOH | Iso-Citrate CH - COOH | HOCH - COOH TCA Cycle – Metabolites 82 Acetyl-CoA 10 4 OAA HOOC-CO-CH2-COOH 1-2-6-1 18 6 CH2-COOH Citrate 1-6-3-1-6-1 COH-COOH CH2-COOH 16 5 α-ketoglutarate HOOC-CH2-CH2-CO-COOH 1-6-6-2-1 12 4 Fumarate HOOC-CH=CH-COOH 1-5-5-1 14 4 Succinate HOOC-CH2-CH2-COOH 1-6-6-1 12 4 Malate HOOC-CH2-CHOH-COOH 1-6-4-1 10 3 Pyruvate CH3-CO-COOH 7-2-1 TCA Cycle – Electron and Carbon Flow Citric acid synthesis during idiophase Pyruvate carboxylase OAA 10 4 Malate 12 4 Fumarate 12 4 Succinate 14 4 Glucose 10 3 Pyruvate 82 Acetyl-CoA Citrate synthase 18 6 18 6 glycolysis 01 24 6 Citrate Isocitrate 16 5 α-ketoglutarate 10 3 + 0 1 + 82 → 10 4 Pyruvate + CO2 + Acetyl-CoA → Citrate TCA Cycle – Electron and Carbon Flow Citric acid synthesis during idiophase 1 mol glucose can result in 1 mol citric acid! 6 electrons need to be disposed of (oxygen) How can citrate be synthesised when pyruvate is not available (e.g. when lipids are the substrate (ß-oxidation))? Citric Acid Synthesis With Lipids as the Substrate Aim: Produce citrate from non-carbohydrate material e.g.: hydrocarbons, fatty acids, ethanol, acetate Problem: ß-oxidation rather than glycolysis is used pyruvate (Pyr carbox.) not available for OAA synthesis Solution: Glyoxylate cycle designed to convert fat into carbohydrates (C2->C3) plant seedlings, microbes, but not animals Citric Acid Synthesis With Lipids as the Substrate Glyoxylate (COH-COOH): • is the second most oxidised biological organic substance • can be fused with acetate to lead to OAA •OAA can then be used for the generation of new citrate 82 + 42 → 12 4 → 10 4 + 20 Acetate + Glyoxylate → Malate → OAA + 2 NADH •What is the reaction that forms glyoxylate ? •Can you think what is the most oxidised organic ? Citric Acid Synthesis With Lipids as the Substrate Glyoxylate is derived from isocitrate lyase reaction: (see glyoxylate cycle) 42 → 12 4 + Isocitrate → Succinate + Glyoxylate 18 6 How can the excretion of citrate be guaranteed when isocitrate is necessary for citrate synthesis? • Example calculation: • Bioreactor: steady state at DO 2 mg/L assume the sat conc to be 8 mg/L • stopped the airflow • OUR = 200 mg/L/h • What would be the max oxidation rate of acetate to CO2 by the reactor when the DO must be at least 1 mg/L? • steady state OUR = OTR • kLa = OTR /(cs – cL) = 200 mg/L/h /(8-2 mg/L)= 33.3 h-1 • OTR at cL = 1 mg/L is OTR = kLa * (8 – 1 mg/L) =233 mg/L/h = 7.3 mmol/L/h • 3.65 mmol of acetate can be oxidised when the reactor runs at DO of 1 mg/L • (MW 32 g/mol) TCA Cycle – Electron and Carbon Flow Citric acid synthesis during trophophase 82 OAA Malate DH Malate Fumarase Fumarate Succinate DH Succinate 10 4 12 4 12 4 14 4 Acetyl-CoA Citrate synthase 18 6 Citrate Aconitase 18 6 Isocitrate 16 5 Isocitrate DH α-ketoglutarate α-ketoglutarate DH How can the cycle continue when citrate is excreted? Citric Acid Synthesis With Lipids as the Substrate Glyoxylate Formation from Isocitrate Lyase 82 OAA 10 4 Acetyl-CoA Citrate synthase 18 6 18 6 14 4 Citrate Aconitase Isocitrate Isocitrate lyase 42 Glyoxylate (CHO-COOH) Citric Acid Synthesis With Lipids as the Substrate Glyoxylate use to lead to OAA via malate 82 82 OAA 10 4 Malate 12 4 14 4 Acetyl-CoA Citrate synthase 18 6 18 6 Citrate Aconitase Isocitrate Isocitrate lyase 42 Glyoxylate (CHO-COOH) How can the excretion of citrate be guaranteed when isocitrate is necessary for citrate synthesis? Citric Acid Synthesis With Lipids as the Substrate (Glyoxylate Cycle) 82 82 OAA 10 4 Malate 12 4 Malate synthase Fumarate 12 4 Succinate 14 4 Acetyl-CoA Citrate synthase 18 6 18 6 Citrate Aconitase Isocitrate Isocitrate lyase 42 Glyoxylate (CHO-COOH) Isocitrate supplies precursors (succinate and glyoxylate) for two OAA, thus allowing the synthesis of 2 citrate, one to be excreted, the second to continue the glyox. cycle. Citric Acid Synthesis With Lipids as the Substrate (Glyoxylate Cycle) Glyoxylate cycle can produce citrate from acetate only: 3 82 → 18 6 + 6 0 3 Acetate → Citrate + 6 H (3 NADH) And again, from the balance we can see that an electron acceptor is needed to accept the excess electrons Citric Acid Production Industrial Problems • Citrate is not a primary metabolite Not formed during exponential growth but under Fe limitation Continuous chemostat culture not suitable unless as multitank system ↑ Na+ → yellow pigment and oxalic acid production • ↑ Fe3+ → ↓ [citric acid], ↑ [oxalic acid], CO2 No iron vessels (not even stainless steel) • Addition of Cu and Zn salts as iron antagonist Typically using Aspergillus niger on sugar media • Use of alcanes and Candida yeast as biocatalyst: + ↑ product yields -low sloubility of substrate (↓ production rate R) -pH must be less than 3.5, otherwise oxalate excretion Citric Acid Production Industrial Problems •Possible reaction of oxalic acid production: 42 → 22 + 20 Glyoxylate → Oxylate + NADH Is anaerobic citric acid production from fats or glucose likely? What is the expected difference in biomass formation during tropho- and idio- phase ? (3ATP/NADH oxidised = 6ATP/O2 used) Interesting biochem: Why is it possible to increase the citric acid output of a glucose degrading culture of A. niger by adding hydrocarbons as a supplement? PEP inhib. ICL phosphoenolpyruvate inhibits isocitrate lyase for good reason: If PEP is there then there is no need to run glyoxylate cycle Citirc Acid Production Process Strain: Aspergillus niger mutants History: • First extracted from immature lemons • 1883 shown microbial metabolite • 1922 nutrient deficiency (Fe) was found to result in high [citrate] Process: • Submerged process (airlift or CSTR) •• pellets formation •• requires well cultivated seed material •• high productivity, low labour costs •• high capital costs, foaming problems Citric Acid Production Process Open vats (still used, cheaper O2 supply) • blow spores onto medium in high purity aluminium vats • allow white mycelium to grow • after pH 5 → 2, drain off liquid and renew (2nd idiophase!) • low capital, high labour costs (Australia) Koji fermentation – Solid surface process (Japan) • similar to shallow trickling filter • support material (wheat bran, etc.) • lower sensitivity of Fe Citirc Acid Production Process Critical process conditions: • Medium: 15 – 25% sucrose solutions (molasses, starch hydrolysates) • 2mg/L Fe3+ required in trophophase • Less than 0.1 mg/L Fe3+ desired in idiophase • Startup pH 5 → drops to pH 2 → low risk of contamination Gluconic Acid Production Process Special property: Complex Ca2+ and Mg2+ ions Use: • Ca gluconate as soluble Ca medication • Sequestering agent in neutral or alkaline solutions E.g. Bottle washing (removes Ca precipitates) • Gluconolactone has latent acidogenic properties Heating gluconolactone →↓ pH because of gluconic acid production (e.g. baking powder, self raising flour) Biochemistry: Glucose oxidation by oxygen with glucose oxidase (biosensors) Glucose + O2 → Gluconate + H2O2 24 6 22 6 → Gluconic Acid Production Process Strain: • Aspergillus niger • Acetobacter suboxidans (also oxidises other alcohol groups to organic acids (e.g. propanol to propionate) → bioconversions Process: submersed Critical process conditions • glucose medium • low temperature (20 °C) • N limitation • neutral pH • absolute sterility Amino Acid Production Glutamate Glutamate and lysine are the most significant commercial amino acids produced by bioprocesses. Strong competition existing from: Lysine: 11% Rest: 2% • chemical synthesis • extraction from animal protein Glutamate is the only mass product Glutamate: 87% Use: Food additive (“flavour enhancer”) Japan, China,… Sold as mono-sodium-glutamate (MSG) Has had bad reputation because of over use. Amino Acid Production Glutamate Biochemistry: • Glycolysis, TCA cycle • reductive amination of α-ketoglutarate (glutamate DH) • block α-ketoglutarate DH • accumulation of α-ketoglutarate • under excess of NH3 → glutamate accumulation • accumulation of glutamate and thus α-ketoglutarate removal requires an anaplerotic sequence to replenish TCA cycle: Glutamate Production 1 10 4 Glucose 10 3 Pyruvate 82 Acetyl-CoA Citrate synthase 12 4 12 4 14 4 α-ketoglutarate DH glycolysis OAA Malate DH Malate Fumarase Fumarate Succinate DH Succinate 24 6 18 6 Citrate Aconitase 18 6 Isocitrate 16 5 Isocitrate DH α-ketoglutarate Glutamate DH 20 NH3 18 5 N Glutamate Amino Acid Production Glutamate • accumulation of glutamate and thus α-ketoglutarate removal requires an anaplerotic sequence to replenish TCA cycle: Malic enzyme: Pyruvate + 2 H + CO2 → Malate 10 3 + 2 0 + 0 1 → 12 4 With hydrocarbons as the substrate: glyoxylate cycle is operable (refer to citric acid production) Glutamate Production 1 OAA Malate DH Malate Fumarase Fumarate Succinate DH Succinate 10 4 Glucose 10 3 Pyruvate 82 Acetyl-CoA Citrate synthase 12 4 12 4 14 4 α-ketoglutarate DH glycolysis 01 20 24 6 18 6 Citrate Aconitase 18 6 Isocitrate 16 5 Isocitrate DH α-ketoglutarate Glutamate DH 20 NH3 18 5 N Glutamate Glutamate Production 2 (Feedback inhibition) Glucose + NH3 → Glutamate + CO2 + 6H 24 6 + N 18 5 N + 0 1 + 6 0 Problem: • glutamate accumulates in the cell causing feedback inhibition (glutamate is not meant to be endproduct (no excretion mechanism)) • Weakened cell membranes are required • Weak membranes are low in unsaturated phospholipids. This can be achieved by: •Biotin deficiency (complex media can not be used) •Addition of saturated fatty acid •Addition of sub lethal doses of penicillin Organisms: • Usually Corynebacterium glutamicium, however • no specific group as long as blocked at a-ketoglutarate DH • Oleate or glycerol auxotrophic mutants used. Growth in the presence of low concentrations of glycerol or oleate Process: • 160 g/L of glucose or acetate medium • pH neutral –>( very prone to contamination) • batch process (revertants (“contamination from inside”, phages, contamination) • 2 -4 days of duration in • submersed process (CSTR) • high oxygen requirement (high KLA) necessary • cooling necessary • combined pH control by NH3 addition allows: •• to optimise N-supply, •• to monitor amino acid production by NH3 used Low oxygen concentration can result in succinate or lactate production (pyruvate hydrogenation)