* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lecture 10
Survey
Document related concepts
History of climate change science wikipedia , lookup
Composition of Mars wikipedia , lookup
Schiehallion experiment wikipedia , lookup
Spherical Earth wikipedia , lookup
History of geomagnetism wikipedia , lookup
Geochemistry wikipedia , lookup
Age of the Earth wikipedia , lookup
Future of Earth wikipedia , lookup
History of geology wikipedia , lookup
History of geodesy wikipedia , lookup
Transcript
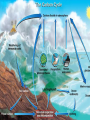
ASTR/GEOL-2040: Search for life in the Universe: Lecture 10 • Sources/sinks of C • Plate tectonics • BIFs and GOE Thanks for the flower! • And the wishes in German! 2 To determine the rock’s age A. Determine its chemical composition B. Identify its mineral structure C. Measure ratios of different isotopes 3 To determine the rock’s age A. Determine its chemical composition B. Identify its mineral structure C. Measure ratios of different isotopes 4 Typo on page 21 last time not 0.102 t/t1/2=log2(1+[40Ar]/0.109 [40K]) 5 Starting values unknown Divide the 2 equations by each other In HW4, just use final equation from lecture Derivation merely for completeness 6 Today • HW4 (due Thursday, Feb 26) – Last before midterm exam (Mar 2) • Carbon dioxide cycle • Plate tectonics • Great Oxidation Event (GOE) 7 On Earth, CO2 is recycled • Sources of CO2 – …. – …. • Sinks of CO2 –… –… 8 On Earth, CO2 is recycled • Sources of CO2 – Animal life on Earth – Oxidation of exhumed CH2O – Other C oxidation (e.g. fire) – Outgassing (volcanoes) – CaCO3 CaO+CO2 or rather • Silicate minerals + CaCO3 new silicate minerals+CO2 9 On Earth, CO2 is recycled • • • • Sources of CO2 …. …. Decarbonation 10 On Earth, CO2 is recycled • • • • Sources of CO2 …. …. Decarbonation 11 Sinks of CO2 • Photosynthetic life (of course) • Acid rain: H2O+CO2 =H2CO3 – Contact with rock: weathering • CaSiO3 +H2CO3 CaCO3 +SiO2 – Calcium carbonate – solid deposit (sea bed) – carbonate rock (limestone) – Details in RGS p.51 12 Sinks of CO2 • Acid rain: H2O+CO2 =H2CO3 – Contact with rock: weathering • CaSiO3 +H2CO3 CaCO3 +SiO2 – Calcium carbonate (solid deposit) – Details in RGS p.51 • On Earth: 170,000 times more CO2 in carbonate rocks than in atmosphere See BS p.139 for details! 13 The CO2 thermostat • Recycling rate sensitive to temperature • CO2 warmer (greenhouse) – More evaporation, more rainfall • Pulling more CO2 out of atmosphere – Weaker greenhouse effect • Negative feedback 14 Feedbacks • Negative feedback – Stable • Positive feedback – Unstable, runaway • Examples? – loudspeaker 15 CO2 thermostat: other way around • Less CO2 cooler – less evaporation, less rainfall • Less removal of CO2 out of atmosphere – greenhouse effect becomes stronger – and it gets warmer again • Again: negative feedback 16 The CO2 thermostat CO2 high, warm, more rain atmospheric CO2 reduced 17 The CO2 thermostat CO2 low, cool, less rain atmospheric CO2 builds up CO2 high, warm, more rain atmospheric CO2 reduced 18 Why so much CO2 on Venus? • Venus: 200,000 times more CO2 than Earth – Remember: Earth 170,000 times more CO2 in carbonate rocks • Venus: no return to carbonate rocks • Why? 19 Why no CO2 return into rocks on Venus? •… • …… • ……….. 20 into rocks Why no CO2 return on Venus? • No water, no rain, no feedback • Yet, volcanic activity Idunn Mons infrared topography enlarged 0.25 Myr old 21 Why did Venus lose its water? • Same reason as for Mars • UV light: H2O H2 + ½ O2 photolysis • H2 lost through thermal escape • Why? 22 Thermal escape? • Escape velocity? – Apollo 8 (Borman, Lowell, Anders) • ½ mve2=GMm/R – ve=(2GM/R)1/2 = 11.2 km/s – ½ mvH2=kBT • H2 is so light 23 Why not on Earth? • Water vapor condenses to rain before too much gets lost • Venus: unable to protect itself • Too hot: H2O also greenhouse gas – Hotter more vapor hotter still • Runaway greenhouse effect 24 Runaway greenhouse effect • Water 25 Earth has far less atmospheric CO2 than Venus because A. Earth was born with less gas B. CO2 was lost in giant impact C. CO2 is locked up in carbonate rocks 26 Earth has far less atmospheric CO2 than Venus because A. Earth was born with less gas B. CO2 was lost in giant impact C. CO2 is locked up in carbonate rocks 27 If Earth had more greenhouse gases in its atmosphere, it would A. Heat up B. Cool off C. Accelerate plate tectonics 28 If Earth had more greenhouse gases in its atmosphere, it would A. Heat up B. Cool off C. Accelerate plate tectonics 29 If Earth had more greenhouse gases in its atmosphere, it would A. Heat up B. Cool off C. Accelerate plate tectonics 30 Earth’s structure • cc 31 We know this from seismology • Crust • Mantle – Upper – Lower • Core – Inner – Outer 32 Asthenosphere: viscous & weak 33 Mantle: 45% by radius • cc 34 Earth’s structure • cc 35 Earth’s structure • cc 36 Plate tectonics • Upwellings (heat) • Subduction • shear 37 Also deep in the pacific & atlantic Rock locations Acasta gneiss • Predictable • Phoenix observed precipitation • But evaporated before reaching ground Isua Barberton Shark Bay Greenstone belt Long, p.211 39 Banded iron formations (BIFs) • Best evidence 40 Banded iron formations (BIFs) • BIF deposits of >2.5Gyr – Estimated mass 3x1016 kg – 30% in hematite (Fe2O3) • How much O? – 2x56+ 3x16=160 – Amount of O: 0.3x3x1016 kg x 48/160 = 3x1015 kg 41 Banded iron formations (BIFs) • Large amounts of O into BIFs early on – Interpretation: Fe minerals dissolved in ocean (e.g., from hydrothermal vents) • Hematite precipitated – Deposited at ocean floor – Atmospheric O2 much came later 42 Snowball Earth events • Geological record – Dramatic climate changes • 0.75 – 0.58 Gyr ago (up to 4) – Global glaciation (snowball Earth) – Mass extinction (CO2 sinks cease) – Explains Fe-rich betw glacial deposits • Similar events earlier in history! 43 Oxidation of Earth atmosphere • Evidence – Oxidized sulfur compounds in rocks – Also reduced sulfur (FeS, sulfide) • First 1 Gyr, only trace amounts of O2 – Plausible source: photosynthesis • Higher levels of O3 – Life under water (protected) 44 Ice ages are different • Temperature drops by a few degrees – More snowfall, down to low latitudes – Last one 10,000 yr ago – Duration: 35 Myr • One contributor: cyclic changes in Earth rotation (tilt 22o-25o) & orbit – Milankovich cycles 45 The great oxidation event (GOE) • 2.3 Gyr ago (weathering saturated) • Diversity of minerals & later life 46 Snowball Earth refers to A. One in a series of deep ice ages that occurred >0.5 Gyr ago B. The idea that Earth would be frozen without greenhouse effect C. Any of the ice ages that occurred in the past few million years 47 Snowball Earth refers to A. One in a series of deep ice ages that occurred >0.5 Gyr ago B. The idea that Earth would be frozen without greenhouse effect C. Any of the ice ages that occurred in the past few million years 48 Next week • • • • Evidence for early life on Earth Oceans 4.4 Gyr ago Significance of 13C isotope Cambrian explosion of life 49