* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Universe Inside of You: Where do the atoms in your body come
Survey
Document related concepts
Formation and evolution of the Solar System wikipedia , lookup
Astronomical spectroscopy wikipedia , lookup
Theoretical astronomy wikipedia , lookup
Star formation wikipedia , lookup
Timeline of astronomy wikipedia , lookup
Chronology of the universe wikipedia , lookup
Type II supernova wikipedia , lookup
Abundance of the chemical elements wikipedia , lookup
Stellar kinematics wikipedia , lookup
Stellar evolution wikipedia , lookup
Future of an expanding universe wikipedia , lookup
Standard solar model wikipedia , lookup
Transcript
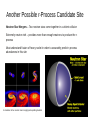
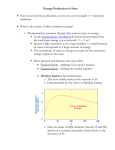
The Universe Inside of You: Where do the atoms in your body come from? Matthew Mumpower University of Notre Dame Thursday June 27th 2013 Nucleosynthesis nu·cle·o·syn·the·sis The formation of new atomic nuclei by nuclear reactions, thought to occur in the interiors of stars and in the early stages of development of the universe. + Nuclear Physics Astrophysics “We are all made of star stuff...” Nuclear Physics Tells Us How nuclei transmute (how one nucleus is turned into another via nuclear reactions) And the energy associated with these various processes. Nuclear reactions are the fuel for stars... Creation of new elements is an energy source of stars (and the sun) Astrophysics Tells Us The properties celestial objects as well as their interactions and time evolution. Stellar Burning Stellar Evolution Our Sun Shines 3.85*1033 erg/s ~ 3.85*1026 Watts for ~4.5 billion years. The sun in x-rays A sun made of coal would only last ~4000 years. Our Sun Shines 3.85*1033 erg/s ~ 3.85*1026 Watts for ~4.5 billion years. The sun in x-rays A sun made of coal would only last ~4000 years. “Billions and billions...” How Old Are The Atoms on Earth? Solar System formed ~4.6 billion years ago from gravitational collapse of interstellar dust cloud. This cloud was generated from previous supernova explosions. Most atoms on Earth are > 4.6 billion years old. Artist's concept of protoplanetary disk “Stuff” In the Solar System Abundance is a quantity denoting how much stuff Heavier elements More stuff Less stuff (number of protons) We know the amount of stuff in the Solar System because we have data from meteorites and the sun's photosphere (outer shell). The sun has 99% of the mass of the solar system – so it is important to understand its composition. “Stuff” In the Solar System Iron Gold A ~ 56 A ~ 197 More stuff Less stuff (number of protons) Creation of the elements did not occur all at the same time nor same place This requires some detective work... What Is The Origin Of The Elements? Lightest Elements: Big Bang Big Bang Nucleosynthesis Created most of the Hydrogen and Helium in the universe. Started within the first 3 minutes of the beginning of the universe. Ended within about 20 minutes due to expanding and cooling. Only ~12 key reactions to take into account! “If you wish to make an apple pie from scratch, you must first invent the universe. ” Cosmic Rays Stellar Burning Processes Stellar Burning Processes Nuclear Fuel Main Products H He He C, O C Ne, Mg Ne O, Mg O Si, S Si Fe Coal T (109 K) Duration (yr) 0.037 8 * 106 0.19 1 * 106 0.87 1 * 103 1.6 0.60 2.0 0.25 3.3 0.03 Energy: Unfavorable Once We Reach Fe Nuclear Fuel Main Products H He He C, O C Ne, Mg Ne O, Mg O Si, S Si Fe T (109 K) Duration (yr) 0.037 ~ 8 * 106 0.19 ~ 1 * 106 0.87 ~ 1 * 103 1.6 ~ 0.60 2.0 ~ 0.25 3.3 < 0.03 p,s,r Processes Particle Accelerators Synthesis of The Heavy Elements Still many open problems... Where are these elements synthesized? (site?) Does fission of the heaviest elements play a role? It is difficult to produce short-lived nuclei that can participate in these processes. What are the masses or half-lives of short-lived nuclei? Question Question #3 #3 How How were were the the elements elements from from iron iron to to uranium uranium made made ?? Chart of Nuclides This system of ordering nuclei can offer a greater insight into the characteristics of isotopes than the better-known periodic table, which shows only elements instead of each of their isotopes. Gold Stable nuclei Known nuclei O No known info Possible r-process path What do lines of constant A = Z + N look like? So How Is Gold Made? Some early attempts tried to use alchemy Turn a base metal into gold or silver A famous example is the philosopher's stone (1590) By a nuclear reaction in the lab For example: 208 Pb + 9Be → 197Au + p + …. Makes gold from lead… at a very slow rate (20,000 nuclide/sec) So How Is Gold Made? Expose iron to a flux of neutrons Recall: not energetically favorable to keep adding protons. There are two possibilities: Capture neutrons very quickly (rapid or r-process). Capture neutrons very slowly (slow or s-process). Neutron Capture / Photo-dissociation Beta Decay The s-Process Timescale for capturing a neutron can be 10 years or longer! Where does this process take place? - In Giant Branch stars There ! “Path” of the nuclear reactions stays close to stability. This process takes millions of years to reach its end point (Bismuth-209). But we see elements heavier than Bi-209 → they must be created in the r-process The s-Process How do we know that nucleosynthesis is going on in red giant stars? Merrill (1952) analyzed the light coming from a red giant and found Technetium (Tc). Tc (Z=43) has no stable isotope (longest ~ 4 million yrs). So since it was found in the star it had to be created there. Another Way To Make Au: The r-Process Capture neutrons very quickly - “rapid” compared to the time it takes to beta-decay. So many neutrons are captured that we push the “path” far from the stable isotopes. No information on nuclear properties of thousands of nuclei that participate in this process because they are too short-lived to be measured. Further complicated because we don't know the location(s) where this process occurs. The location must be able to provide a lot of neutrons! Event lasts ~10 seconds Neutron Capture / Photo-dissociation Beta Decay One Possible r-Process Candidate Site Supernova – End of the life of a massive star (remember the iron core?) Extremely luminous - burst of radiation that can outshine host galaxy for several weeks expelling most of the star's material During this short interval a supernova can radiate as much energy as the Sun is expected to emit over its entire life span. Can it produce neutron rich material?... Another Possible r-Process Candidate Site Neutron Star Mergers – Two neutron stars come together in a violent collision Extremely neutron rich – provides more than enough neutrons to produce the rprocess Must understand fission of heavy nuclei in order to accurately predict r-process abundances in this site A simulation of two neutron stars merging and expelling material Some Clues From Astrophysics We look to the galactic halo and analyze the abundances of very old “halo” stars. We find that the composition of these stars is similar to the Solar System. This means that whatever processes are at work creating the heavy elements are fairly universal. Maybe It's Both? Could supernova and neutron star mergers both be responsible for the r-process? Look at Europium (a known r-process element) in stars. There seems to be two components. More r-process material r-Process Enrichment [Eu/Fe] Older stars 5.0 4.0 3.0 2.0 1.0 0.0 -1.0 -6.0 -5.0 -4.0 -3.0 -2.0 Metallicity -1.0 [Fe/H] 0.0 1.0 Data from the Stellar Abundances for Galactic Archeology (SAGA) database A Simple r-Process Calculation nuclear physics inputs (Sn, β-rates, n-cap rates, … ) reaction network Environment conditions (temperature, density, ... ) abundances Other Nucleosynthesis Processes How to make the proton-rich heavy elements? Proton capture (p-process) and rapid proton capture (rp-process) Other astrophysical environments? More investigation needed... More movies of nucleosynthesis: http://www.jinaweb.org/html/gallery_scimovies.html Summary ● Carl Sagan has some good quotes ● Solar system is about 4.5 billion years old ● Sun will “live” for another ~5 billion years ● “Heavy” Elements are created by stars ● ● ● Different processes occur in different sites and produce different isotopes Nuclear physics is import to understanding abundances Window of a church in Wixhausen, Germany Even German churches like nucleosynthesis “... and billions...”