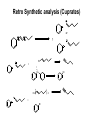* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Ch 26 C-C bond formation
Aromaticity wikipedia , lookup
Persistent carbene wikipedia , lookup
Fischer–Tropsch process wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Elias James Corey wikipedia , lookup
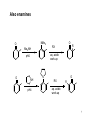
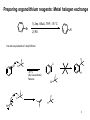
Marcus theory wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Discodermolide wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
George S. Hammond wikipedia , lookup
Asymmetric induction wikipedia , lookup
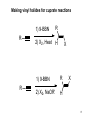
Physical organic chemistry wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Ene reaction wikipedia , lookup
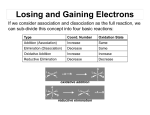
Baylis–Hillman reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Stille reaction wikipedia , lookup
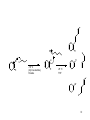
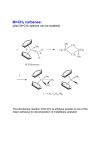
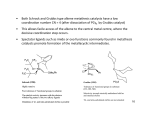
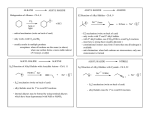
C-C Bond formation Chapter 26 1 Carbon–Carbon Bond Forming Reactions • To form the carbon skeletons of complex molecules, organic chemists need an extensive repertoire of carbon–carbon bond forming reactions. • We have earlier looked at reactions of organometallic reagents such as Grignard, organolithium and organocuprate reagents with carbonyl and other substrates to form larger molecules. • The focus of this chapter will be on additional carbon–carbon bond forming reactions which utilize a variety of starting materials and conceptually different reactions. • Three such reactions involve coupling of an organic halide with an organometallic reagent or alkene: (1) Organocuprate coupling reactions, (2) Suzuki reaction, and (3) Heck reaction. 2 3 4 5 Also enolates O R R X O O LDA, -78 °C O CH2O THF HO RN O O RN O Br O O O O 6 Also enamines NMe2 O RX Me2NH R aq. acidic work-up pH4 O O NH pH4 N O RX R aq. acidic work-up 7 Preparing organolithium reagents: Metal halogen exchange 1) 2eq. t-BuLi, THF, -78 °C Br R 2) RX Use two equivalents of t-butyl lithium Br Li Li -78 °C (dry ice-acetone) Hexane H Br H Li H Br 8 Troublesome side reactions: Alkylation with alkylbromides from organolithium preparation Br Br Li Li -78 °C (dry ice-acetone) Hexane H H H Br Li Elimination reactions with alkylbromides from organolithium preparation or intended alkyl halide Br RT H Li 9 H Br Br H Li Li -78 °C (dry ice-acetone) Hexane H H -78 °C THF Br Li 10 Organolithium pKa’s pKa 70 H tert-butyl lithium Li pKa 65 H Li sec-butyl lithium Li n-butyl lithium pKa 62 H pKa 60 methyl lithium H3C Li H3C H pKa 43 vinyl lithium Li H pKa 42 H Li phenyl lithium 11 More reactive Less reactive RLi > RNa > RK > RMgX > R2Zn > R2CuLi > R3Al > R3B > R4Si MgBr Li MgBr2 + LiBr 12 Coupling Reactions of Organocuprates • Organocuprate reagents react with a variety of functional groups including acid chlorides, epoxides and ,unsaturated carbonyl compounds. • Organocuprate reagents also react with organic halides R′–X to form coupling products R–R′ that contain a new C–C bond. • Only one R group of the organocuprate is transferred to form the product, while the other becomes part of the RCu, a reaction product. 13 General Features of Organocuprate Coupling Reactions • Methyl, 1°, cyclic 2°, vinyl, and aryl halides can be used. • Reactions with vinyl halides are stereospecific. • The halogen (X) may be Cl, Br, or I. • Tertiary (3°) halides are too sterically hindered to react. 14 Making vinyl halides for cuprate reactions R R 1) 9-BBN R 2) X2, Heat H X 1) 9-BBN R 2) X2, NaOR' H X 15 Coupling to Form Hydrocarbons • Since organocuprate reagents are prepared in two steps from alkyl halides (RX), this method ultimately converts two organic halides (RX and R′X) into a hydrocarbon R–R′ with a new carbon–carbon bond. • This means that using this methodology, a given hydrocarbon can often be made by two different routes. 16 Retro Synthetic analysis (Cuprates) “….the grand thing is to be able to reason backwards. That is a very useful accomplishment, and a very easy one, but people do not practice it much.” Sherlock Holmes in “A Study in Scarlet” Br CuI R Break into equal size fragments at branch points or appropriately adjacent to functionality ? Br Retro Synthetic analysis (Cuprates) or ? Br = I Cu Br 2 ICu = Br Organopalladium Mediated Reactions Suzuki Reaction R BY2 + Pd(PPh3)4 R' X R R' Base R'-I > R'-OTf > R'-Br >> R'-Cl R & R' = alkyl, aryl, alkenyl, alkynyl Heck Reaction R X R' H R Pd(0) R' Base -HX R = aryl, alkenyl or benzyl X = Cl, Br, I, O O S CF3 O 19 Organopalladium Compounds • During a reaction, Pd is coordinated to a variety of groups called ligands, which donate electron density to (or sometimes withdraw electron density from) the metal. • A common electron donating ligand is phosphine, some derivatives of which are shown: 20 Organopalladium Compounds • Organopalladium compounds are generally prepared in situ during the course of a reaction, from another palladium reagent such as Pd(OAc)2 or Pd(PPh3)4. • “Ac” is the abbreviation for the acetyl group, CH3C=O, so OAc is the abbreviation for CH3CO2−. • In most useful reactions, only a catalytic amount of Pd reagent is used. • Two common processes, called oxidative addition and reductive elimination, dominate many reactions of palladium compounds. 21 Oxidative Addition and Reductive Elimination 22 Details of the Suzuki Reaction • The Suzuki reaction is a palladium-catalyzed coupling of an organic halide (R′X) with an organoborane (RBY2) to form a product (R–R′) with a new C–C bond. • Pd(PPh3)4 is the typical palladium catalyst. • The reaction is carried out in the presence of a base such as NaOH or NaOCH2CH3. • Vinyl or aryl halides are most often used, and the halogen is usually Br or I. • The Suzuki reaction is completely stereospecific. 23 Examples of the Suzuki Reaction 24 Organoboranes in Suzuki Reaction • Two types of organoboranes can be used in the Suzuki reaction: vinylboranes and arylboranes. • Vinylboranes, which have a boron atom bonded to a carbon– carbon double bond, are prepared by hydroboration using catecholborane, a commercially available reagent. • Hydroboration adds H and B in a syn fashion to form a trans vinylborane. • With terminal alkynes, hydroboration always places the boron atom on the less substituted terminal carbon. 25 Preparation of Arylboranes • Arylboranes, which have a boron atom bonded to a benzene ring, are prepared from organolithium reagents by reaction with trimethyl borate [B(OCH3)3]. 26 27 Synthesis Using the Suzuki Reaction • The Suzuki reaction was a key step in the synthesis of bombykol, the sex pheromone of the female silkworm moth. • The synthesis of humulene illustrates that an intramolecular Suzuki reaction can form a ring. Figure 26.2 28 Retrosynthetic Analysis of Suzuki Reaction R" R" Aryl-Aryl links R" (R"O)2B Br or Br R B(OR")2 R R Aryl-Alkenyl links R" (R"O)2B R" Br R B(OR")2 or R R Alkenyl-Alkenyl links (R"O)2B R" R R" Br Br R R" Br or R" B(OR")2 R 29 Synthesis using Suzuki Coupling reaction in Loy Lab B(OH)2 2 S S Pd(PPh3)4 1 Na2CO3 Br (HO)2B dioxane/H2O S 1,4-di(thiophen-3-yl)benzene New monomers for making flame resistant polymers 30 The Heck Reaction • The Heck reaction is a Pd-catalyzed coupling of a vinyl or aryl halide with an alkene to form a more highly substituted alkene with a new C–C bond. • One H atom of the alkene starting material is replaced by the R’ group of the vinyl or aryl halide. • Palladium(II) acetate [Pd(OAc)2] in the presence of a triarylphosphine [P(o-tolyl)3] is the typical catalyst. • The reaction is carried out in the presence of a base such as triethylamine. 31 The Heck Reaction • The alkene component is typically ethylene or a monosubstituted alkene (CH2=CHZ). • The halogen is typically Br or I. • When Z=Ph, COOR or CN in a monosubstituted alkene, the new C–C bond is formed on the less substituted carbon to afford a trans alkene. • When a vinyl halide is used as the organic halide, the reaction is stereospecific. 32 Examples of the Heck Reaction 33 Using the Heck Reaction in Synthesis • To use the Heck reaction in synthesis, you must determine what alkene and what organic halide are needed to prepare a given compound. • To work backwards, locate the double bond with the aryl, COOR, or CN substituent, and break the molecule into two components at the end of the C=C not bonded to one of these substituents. 34 35 Retrosynthetic Analysis of Heck Reaction Br R" R R" R Advantage over Suzuki Coupling: fewer steps (No boranic ester is needed) 36 Heck Reaction in Loy Lab Si(OEt)3 Br 1 eq. Pd(OAc)2 Br Et3N 2 eq. (EtO)3Si (EtO)3Si Dye for making fluorescent nanoparticles 37 Carbenes • A carbene, R2C:, is a neutral reactive intermediate that contains a divalent carbon surrounded by six electrons—the lone pair, and two each from the two R groups. • These three groups make the carbene carbon sp2 hybridized, with a vacant p orbital extending above and below the plane containing the C and the two R groups. • The lone pair occupies an sp2 hybrid orbital. Singlet carbene 38 Dihalocarbenes • Dihalocarbenes, :CX2, are especially useful reactive intermediates since they are readily prepared from trihalomethanes (CHX3) by reaction with strong base. • For example, treatment of chloroform (CHCl3) with KOC(CH3)3 forms dichlorocarbene, :CCl2. • Dichlorocarbene is formed by a two-step process that results in the elimination of the elements of H and Cl from the same carbon. • Loss of the two elements from the same carbon is called elimination. 39 40 Dihalocarbenes in Cyclopropane Synthesis • Since dihalocarbenes are electrophiles, they readily react with double bonds to afford cyclopropanes, forming two new carbon–carbon bonds. 41 Carbene Addition to Alkenes • Cyclopropanation is a concerted reaction, so both bonds are formed in a single step. • Carbene addition occurs in a syn fashion from either side of the planer double bond. • Carbene addition is a stereospecific reaction, since cis and trans alkenes yield different stereoisomers as products. 42 Methylene, The Simplest Carbene • Methylene, :CH2, is readily prepared by heating diazomethane, which decomposes and loses nitrogen. • The reaction of methylene produced in this manner with an alkene often leads to a complex mixture of products. • Thus the reaction cannot be reliably used for cylcopropane synthesis. 43 Polymerization of carbenes: Thermal of Lewis acid catalyzed H2C N N H H BF3 -N2 n Noble metal catalyzed O OEt L2PdCl2 CO2Et CO2Et CO2Et CO2Et N N -N2 EtO2C EtO2C EtO2C 44 Polymerization of carbenes: Mechanism Cl Initiation O O H3N EtO Pd EtO N -N2 N N O Cl OEt H3N Pd Cl N Propagation O H3N EtO OEt O EtO Cl Pd Pd Cl O N O H3N H3N N O Cl Pd n OEt O OEt Carbene insertions OEt Termination EtO H3N O Cl Pd n O OEt EtO H3N O Cl n O Pd(0) OEt 45 The Simmons–Smith Reaction • Nonhalogenated cyclopropanes can be prepared by the reaction of an alkene with diiodomethane, CH2I2, in the presence of a copper-activated zinc reagent called zinc– copper couple [Zn(Cu)]. • This is known as the Simmons–Smith reaction. • The reaction is stereospecific. 46 47 Alkene Metathesis • Alkene (olefin) metathesis is a reaction between two alkene molecules that results in the interchange of the carbons of their double bonds. • Two and two bonds are broken and two new and two new bonds are formed. 48 Catalysts for Metathesis • Olefin metathesis occurs in the presence of a complex transition metal catalyst that contains a carbon–metal double bond. • The metal is typically ruthenium (Ru), tungsten (W), or molybdenum (Mo). • In a widely used catalyst called Grubbs catalyst, the metal is Ru. • Metathesis catalysts are compatible with the presence of many functional groups (such as OH, OR, and C=O). 49 Usefulness of Metathesis Reactions • Because olefin metathesis is an equilibrium process and with many alkene substrates yields a mixture of starting material and two or more alkene products, it is useless for preparative processes. • However, with terminal alkenes, one metathesis product is ethylene gas (CH2=CH2), which escapes from the reaction mixture and drives the equilibrium to the right. • Thus, monosubstituted alkenes (RCH=CH2) and 2,2disubstituted alkenes (R2C=CH2) are excellent metathesis substrates because high yields of a single alkene product are obtained. 50 Examples of Alkene Metathesis 51 Drawing the Products of Alkene Metathesis Figure 26.2 52 53 Retrosynthetic Analysis of Metathesis Reaction R R R' R' Are precursors available? Will cross reaction ( R ≠ R’) be dominant 54 Br Br Br ? ? 55 Metathesis in Loy Lab 2 eq. Grubb's 2nd Gen Cat. CH2Cl2 2 eq. CH2=CH2 Grubbs 2nd Generation Catalyst CH3 H3C N N H 3C H3C CH3 CH3 Cl Cl Ru Ph P 56 Ring Closing Metathesis (RCM) • When a diene is used as a starting material, ring closure occurs. • These reactions are typically run in very dilute solution so that the reactive ends of the same molecule have a higher probability of finding each other. • High dilution favors intramolecular rather than intermolecular metathesis. 57 Ring Closing Metathesis (RCM) in Synthesis • Epothilone A is a promising anticancer agent that was first isolated from soil bacteria from the banks of the Zambezi River in South Africa. • Sch38516 is an antiviral agent active against influenza A. 58 Ring Opening Metathesis Polymerization n Grubbs Cat. DCM n + n H2C CH2 Poly(norbornene) 59 Ring Opening Metathesis Polymerization Of benzvalene Benzvalene is a less stable isomer of benzene ΔH = -282 kJ/mole Metathesis catalyst heat n n 60 General synthesis strategies 1) Build carbon framework with enolate reactions and other C-C bond building reactions (Suzuki, Heck, Metathesis, Cuprates, Diels-Alder, & more.) 1) Use functionalities to activate C-C bond building chemistry 2) Functional group conversions to provide desired organic substituents 61 Directions in Synthesis 1) Regio- and stereochemical specificity 1) More atom efficiency (less waste). 1) Reactions using aqueous or non-toxic solvents (H2O, alcohols) 2) Reactions using less hazardous starting materials 62 Some practice: How would you make 1,7-diphenylheptane Br 63 Some practice: How would you make (Z)-cyclooctene 64