* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Newly Approved Anticoagulants
Neuropharmacology wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Prescription costs wikipedia , lookup
Adherence (medicine) wikipedia , lookup
Drug interaction wikipedia , lookup
Metalloprotease inhibitor wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Discovery and development of cyclooxygenase 2 inhibitors wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Theralizumab wikipedia , lookup
Dydrogesterone wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Discovery and development of direct Xa inhibitors wikipedia , lookup
Discovery and development of direct thrombin inhibitors wikipedia , lookup
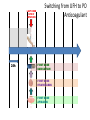
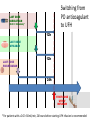
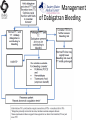
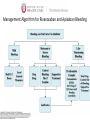
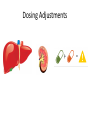
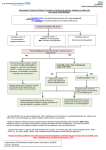
Novel Oral Anticoagulants (NOAC) Dan Moellentin, PharmD, BCPS, Associate Professor Husson University Strokes in Atrial Fibrillation • 1/5 of strokes are caused by a. fib • 1/3 cardiac arrhythmias hospitalizations • 2 million Americans affected by a. fib ~50% are anti-coagulated? What would be the best anticoagulation Fast onset Antidote Brief History of Anticoagulation in Atrial Fibrillation (a. fib) Warfarin was approved in 1954 • INR wasn’t created by the World Health Organization until the 1980’s Until 2009, warfarin was considered the gold standard for anticoagulation in a. fib. Now there are three new oral anticoagulants FDA approved for anticoagulation in a. fib Vitamin K antagonist Drug • Warfarin – Blocks vitamin K dependent clotting factors creation, by inhibiting the vitamin vitamin K epoxide reductase complex 1 (VKORC1) – Vitamin K epoxside is responsible for the regeneration of vitamin K in the vitamin K cycle by blocking VKORC1 – Inhibit vitamin K epoxide reducatase thus interfering with levels of II, V, VII, and IX LOOK UP LEVELs## Patient Case #1 • The majority of warfarin is elimination via metabolism – Clinical example---pt post bowel resection-- Genetic Variance in 2C9 • Patients with 2C9 gene variance require lower doses for therapeutic INR and are at increased risk of bleeding • Activity of 2C19 and 2C9 are genetically determined, 2C9 is more important as it metabolizes the most potent enantiomer, Swarfarin. – 20% of Caucasians, 5% Africa Americans (AA), 2% Asians have a least 1 gene variant Genetic variations in Vitamin K epoxide reductase complex 1 (VKORC1) Loss of function, results in increased sensitivity to warfarin and increased potential for drug interactions because VKORC1 does not function normally to produce vitamin K dependent clotting factors (II, VII, IX, X, proteins C and S) – 37% of Caucasians, 14% of AAs, 89% of Asians have at least one variant of VKORC1 Clinical Significance of Genetic Testing before Warfarin initiation • Not all centers have on-site testing • Genetic testing costs about $200 • Is it paid for by Medicare part D or private insurance? • Adding a few extra days to hospitalization is expensive • Is it only useful in guiding early dosing, ie. does it just save one dose? • “Drug interactions with warfarin may negate the influence of genetic variations and must be considered”. Cytochrome P450 Interactions • Drugs that inhibit 2C9 – Bactrim, fluconazole, amiodarone, fluvastatin, lovastatin, voriconazole, metronidazole, acetaminophen 2g daily for more than a week, cimetidine 400mg daily or less, omeprazole at higher doses, • 3A4 Strong Inhibitors – Erythromycin, diltiazem, nefazadone, verapamil Drugs Affecting Absorption Case #1 Cholestyramine or Colestipol • Get Dan to write case Warfarin MOA: Vitamin K Antagonist (VKA) Initial dose: 10 mg PO x 2 days, then dose based on INR Half-life: 40 hours Monitoring: • If INR is ≤ 0.5 from the target range, keep the same dose and check INR in 1-2 weeks. • Once INR is stabilized, one INR reading up to every 12 weeks is adequate. Only One is Also Approved for DVT/PE Treatment/Prophylaxis Rivaroxaban • “Treatment of deep vein thrombosis (DVT) pulmonary embolism” • “Reduction in the risk of recurrence of DVT” • “PE for the prophylaxis of DVT, which may lead to PE in patients undergoing knee or hip replacement surgery” Newly Approved PO anticoagulants • All 3 are FDA indicated for – “reducing the risk of stroke and systemic embolism in patients with non-valvular atrial fibrillation” • Direct Thrombin Inhibitor – Pradaxa® (dabigatran) • Approved in 2010 • Xa Inhibitors – Xarelto® (rivaroxaban) • Approved in 2011 – Eliquis® (Apixaban) • Approved in 2013 Medication Warfarin Dabigatran Rivaroxaban Apixaban Class VKA Direct Thrombin Inhibitor Xa Inhibitor Xa Inhibitor Half-life 40 hours 12 to 17 hours 5 to 9 hours 12 hours Dosing for A. fib 10mg x 2 days, then 150 mg twice daily based on INR 20 mg once daily 5mg twice daily Majority of Elimination Hepatic CYP • 2C9 80% Renal Renal 66% 25% Hepatic • CYP 3A4 27% Renal Plasma Protein Binding 99% 35% 92 – 95% 87% Dialyzable No 60% No No Data Medication Warfarin Dabigatran Rivaroxaban Apixaban Renal Dose Adjustments Based on INR CrCL 15-30 mL/min • 75 mg twice daily CrCL 15-50 mL/min • 15 mg once daily If ≥2 of the following • ≥ 80 yoa • ≤ 60 kg • ≥ 1.5 mg/dL 2.5 mg twice daily • CrCL < 15 mL/min • Moderate to Severe hepatic impairment • Prosthetic heart valves Hepatic Dose Adjustments None Avoid Use • CrCL < 15 mL/min • Mechanical prosthetic heart valves • Bioprosthetic heart valves LAST DOSE UFH IV INFUSION 24h Switching from UFH to PO Anticoagulant FIRST DOSE DABIGATRAN FIRST DOSE RIVAROXABAN FIRST DOSE APIXABAN Switching from PO anticoagulant to UFH LAST DOSE DABIGATRAN (CrCl > 30mL/min)* 12h LAST DOSE APIXABAN LAST DOSE RIVAROXABAN 12h 24h FIRST DOSE UFH IV INFUSION *For patients with a CrCl <30mL/min, 24 hours before starting UFH infusion is recommended Coagulation Cascade ® Pradaxa (dabigatran) MOA: Direct Thrombin Inhibitor Half-life: 12 to 17 hours Dosing: • 150mg PO twice daily • CrCL 15-30 mL/min – 75 mg twice daily Adverse Effects: • Dyspepsia • GI bleeding Prescribing Information Drug Interactions for Dabigatran • USA – Amiodarone, dronedarone, quinidine, verapamil, clarithromycin, and ketoconazole, rifampin. • Canada – Amiodarone, dronedarone, verapamil, quinidine, rifampicin – Contraindications: Ketoconazole • EMA – Strong P-gp inhibitors: Amiodarone,, verapamil, quinidine, clarithromycin – P-gp inducers: rifampicin – Contraindications: Ketoconazole, itraconazole, tacrolimus, dronedarone, cyclosporine Xarelto® (rivaroxaban) MOA: Xa Inhibitor Half-life: 5 to 9 hours Dosing: • A.Fib: – CrCL >50 mL/min • 20 mg once daily – CrCL 15-50 mL/min • 15 mg once daily Adverse Effects Eliquis® (apixaban) MOA: Xa Inhibitor Half-life: 12 hours Dosing: 5mg PO twice daily • 2.5 mg twice daily – If ≥2 of the following • ≥ 80 yoa • ≤ 60 kg • ≥ 1.5 mg/dL Adverse Effects: Pt casen #1 dabigitran plus amiodarone • Pgp- problem is fda lit P-glycoproteins (P-gp)? P-gps are efflux drug transporters P-gp P-gp function is to excrete or protect the tissues from xenobiotic absorption. • Also referred as PGY1, multidrug resistance protein-1 (MDR1). • Member of the adenosine triphosphate (ATP) binding cassette (ABC) gene • ABCB1 is the gene that encodes for P-gp P-gp in the body P-gp Substrates, Inhibitor, Inducer P-gp Substrates P-gp Inhibitors P-gp Inducers Amiodarone Amiodarone Prazosin Atorvastatin Atorvastatin St. John’s Wort Dabigatran Carvedilol Digoxin Diltiazem Diltiazem Dipyridamole Lidocaine Dronedarone Lovastatin Lidocaine Nadolol Lovastatin Nicardipine Quinidine Pravastatin Propranolol Propranolol NIcardipine Quinidine NIfedipine Rivaroxaban Simvastatin Simvastatin Verapamil Verapamil Clinically Significant P-gp Inhibitors Captopril Carvedilol (Males) Ranolazine Quinidine Cmaxi ,ss/Cmax,ss Ratio Quinidine AUCi/AUC Ratio Verapamil Amiodarone (400 mg x 5 wks) Amiodarone (800mg x 1 wk) Ranolazine 0 0.5 1 1.25 1.5 2 2.5 3 Clinically Significant P-gp Inhibitor • ≥ 1.25 ratio for AUCi/AUC Ratio and Cmaxi,ss/Cmax,ss Ratio Source http://www.medscape.org/viewarticle/467503 P-gp Substrates P-gp Inhibitors P-gp Inducers Digoxin Amiodarone Amiodarone Diltiazem Atorvastatin Diltiazem Quinidine Diltiazem Nicardipine Verapamil Felodipine Nifedipine Lidocaine Verapamil Nicardipine Quinidine Verapamil Dabigatran P-gp interactions P-gp Substrate Dabigatran P-gp Inhibitor Dronedarone Amiordarone Quinidine Verapamil Dabigatran + P-gp inhibitor • Results in increased dabigatran concentrations and adverse events Rivaroxaban P-gp Interactions Removed from the intestines by P-gp Don’t use with rivaroxaban Greater risk with decreased renal function Strong P-gp and 3A4 Inhibitors Moderate P-gp and 3A4 Inhibitors Ketoconazole Itraconazole Amiodarone Dronedarone Diltiazem Verapamil Felodipine Quinidine Ranolazine Apixaban P-gp Interactions Apixaban • ↑Apixaban conc. – Itraconazole, ketoconazole, ritonavir, and clarithromycin Case #3 rivaroxaban Time in Therapeutic Range (TTR) for Warfarin • TTR – % of days that the patients INR is from 2.0 to 3.0 • “TTR in all the other modern warfarin-controlled studies of anticoagulatns ranged from 63% to 73%” – Phase III Trials’ TTR % » RELY (Dabigatran) • 64% » Rocket AF (Rivaroxaban) • 55% » Aristotle (Apixaban) • 62.2% Is Rivaroxaban Dosing Appropriate? Half-life: 5 to 9 hours Non-valvular A. Fib • 20 mg PO once daily Phase II trials suggested that 10 mg twice daily could be safer than 20 mg once daily FDA WORDING • INADEQUATE ON DABIGITRAN • DOSING LIKELY WRONG FOR DVT PROP ON RIVAROX APIXIBAN DOSING CORRECTNESS IS UNKNOWN • WHAT IS DIFFERENT ON LAST 2 DRUGS IS THAT DECISION FOR DOSE ADJUSTMENT IS NOW A PROVIDER JUDGEMENT BASED ON 3A4 AND PGP No NOAC Are Approved for ACS Medications Phase II Trials Clinically Significant Bleeding P < 0.001 Hazard Ratio Dabigatran RE-DEEM 75 mg: 4.3%, 150 mg: 7.8%, 150 mg 4.27 (95 % CI 1.86 -9.81) Rivaroxaban ATLAS ACSTIMI 46 Percentages were not listed, P <0.0001 10 mg: 3.35 15 mg: 3.6 20 mg: 5.06 Apixaban APPRAISE 2.5 mg BID: 3.2% 10 mg QD: 5.5% 10 mg BID and 20 mg QD were stopped because of too many bleeding episodes 2.5 mg BID: 1.78 10 mg QD: 2.45 Safety of Triple Antithrombotic Therapy in ACS Patients Single Antiplatelet + NOAC • Aspirin – ↓’d the occurrence of major adverse cardiovascular events (MACE) • HR 0.70, (95% CI 0.59- 0.84) – BUT ↑’d clinically relevant bleeding • HR 1.79 (95% CI 1.54-2.09) Dual Antiplatelet +NOAC • Aspirin + Clopidogrel – ↓’d the occurrence of MACE • HR 0.87 (95% CI 0.80-0.95) – More than two fold ↑ in clinically relevant bleeding • HR 2.34 (95% CI 2.06-2.66) Risk Assessment Physicians will need assess the patient’s risk for • Thromboembolism – CHADS2 Score ≥ 2 = probably require triple antithrombotic therapy • Hemorrhaging “ Clinicians should strive to limit the use of dual antiplatelet agents with concurrent antithrombotics in patients who are at the highest risk for thromoembolic events and ensure that these patients are instructed to report any signs and sympotms of bleeding or recurrent thrombosis” Dager W PharmD PCPS (AQ Cardiology) Reversal and Monitoring of nOAC Reversal of Dabigatran Management of Dabigatran Bleeding Management Algorithm for Dabigatran Bleeding Management for bleeding from Xa Inhibitors Management Algorithm for Rivaroxaban and Apixaban Bleeding Reversal of Dabigatran • Since dabigatran directly blocks thrombin and does not decrease the coagulation factors, using coagulation factors like, PCC, FFP is NOT expected to be completely effective as a reversal agent. • The best method of reversing dabigatran is to be excreted out by the kidneys. What are PCCs? Prothrombin Complex Concentrates (PCC) Note: No PCC is FDA approved for reversing anticoagulants Three Factor (II, IX, X) FDA approved – Profilnine® SD – Bebulin® VH Four Factor (II, VII, IX, X) • Unactivated (NOT FDA Approved) – Beriplex P/N – Octaplex • Activated – Feiba NF (Factor VII blocker bypassing function) Factor VII Blockers) • Only factor VII is activated Antibody Antidote for Dabigatran? Boehringer Ingelheim: manufacture dabigatran (Pradaxa®) • Currently developing and studying pre-clinically a humanized antibody fragment (Fab) that could be used as a reversal agent for dabigatran. – Rats were given dabigatran and “there was a rapid, dose-dependent decrease in bleeding time after IV injection of Fab” – Additionally, the Fab reversed clotting ex vivo as well. Reversal of Rivaroxaban? Phase I Trials • Four Factor PCC (Factor II, VII, IX and X) – After 3 days of rivaroxaban and one dose of PCC PT and ETP was statistically significantly decreased • PRT4445 – New recombinant protein that blocks Xa inhibitors by serving as a decoy. – The manufacters of PRT4445 has report its safe and tolerable and claims it reverses Xa inhibitors in 5 minutes and last 3 hours, however, the study isn’t available online yet. So What is the “Right” Answer? Pre-clinical studies Studies showing PCC only improves labs not bleeding in apixaban and rivaroxaban Warfarin Reversal • Is warfarin really “reversed?” • Example intracranial hemorrhage (ICH)…. Gastrointestinal Bleeding • Higher risk for DVT/PE treatment vs. DVT/PE prophylaxis after hip/knee replacement surgery – Rivaroxaban 10 mg once daily • Hip: 35 days • Knee: 12 days – Maybe a dose-dependent effect • Dabigatran and rivaroxaban – Higher risk than apixaban Numbers of post-marketing cases of ICH and retroperitoneal hemorrhages Apixaban Rivaroxaban Dabigatran Post Marketing • Incidence of stroke, major bleeding, ICH Post Marketing • Bleeding Risk with Dabigatran in the Frail Elderly NEJM http://www.nejm.org/doi/full/10.1056/NEJMc 1112874 Surgery Dabigatran • CrCL ≥ 50mL/min stop 1-2 days prior to surgery • CrCL < 50mL/min stop 3-5 days prior to surgery Rivaroxaban • Stop ≥ 48 hours prior to surgery for moderate to severe bleeding risk • Stop ≥ 24 hours prior to surgery for normal risk of bleeding Apixaban • Stop ≥ 48 hours prior to surgery for moderate to severe bleeding risk • Stop ≥ 24 hours prior to surgery to low risk of bleeding Monitoring Dabigatran • Useful in establishing if drug in present or not – Normal aPTT: barely any dabigatran present – TT (Thrombin Time): linear dose-response curve for dabigatran, NOT after steady-state. Rivaroxaban and Apixaban?? • aPTT • PT/INR • Anti-Xa Level Monitoring for Dabigatran Monitoring on Xa Inhibitors (Rivaroxaban & Apixaban) Why Not Just Use Anti-Xa Assays to Monitor Xa Inhibitors Dosing Adjustments Hepatic Impairment Apixaban Mild • No dose reduction is required Moderate • No data available Severe • Not recommended Hepatic Impairment Rivaroxban • Don’t use – Moderate and severe impairment – Liver disease that involves bleeding disorders Renal Impairment Apixaban • 2.5 mg twice daily if 2 or more of the following exist 1. ≥ 80 years of age 2. Weight ≤ 60 kg 3. Scr ≥ 1.5 mg/dL Currently per package insert there is “no date inform use in patients with CrCl <15 min/ml or on dialysis” Renal Impairment Rivaroxaban • A. fib – CrCl >50 ml/min • 20 mg daily with dinner – CrCl 15-50 ml/min • 15 mg daily with dinner – Don’t use if CrCl <15ml/min • Treatment of DVT, PE and decreasing the risk of another DVT and PE – Don’t use if CrCl <30 ml/min Renal Impairment • • • • Dabigatran 80% excreted renally ~60% is removed by dialysis Half-life > 24 hours during renal impairment CrCL – 15 to 30mL/min • 75mg twice daily – < 15mL/min or on dialysis : • Don’t use Institute for Safe Medication Practices (ISMP) ISMP • Federally certified, nonprofit organization dedicated to patient safety by – Education on safe medication use – Compile and examine medication errors, side effects, and near misses. – Distribute up to date medication safety news, provide ways of preventing errors, and tools to decrease risks ISMP 1st Quarter Watch 2012 Rivaroxaban • 356 Reports of severe, damaging, or deadly adverse effects – 44%: thrombotic events • Majority: PE • Appearing in prophylaxis for DVT/PE post surgery and in younger patents than dabigatran adverse effects » (average age 66) • Dabigatran – Bleeding was appearing in elderly patients (average 80 years old) ISMP 2012 2nd Quarter Watch Dabigatran – The odds of recorded death from dabigatran were about five fold greater than warfarin. Rivaroxaban – The odds of recorded death from rivaroxaban were about less than two times greater than warfarin. – 10 mg once daily vs. 20 mg once daily • 10 mg once daily – 7 fold greater odds of a recoded emoblicthrombotic occurrence. FDA Draft Briefing Document for the Cardiovascular and Renal Drugs Advisory committee (CRDAC) for Rivaroxaban • “Mean compliance rates ranged from a low of 95.2% (North America, warfarin arm) to a high of 97.1% (Eastern Europe, rivaroxaban arm)” • Day 1-30: % of warfarin naïve patients with an INR in 23 range – 30.68% • It wasn’t until day 181-360 that over 50% warfarin naïve patients • By Day 181-360 50% of warfarin naïve patients had an INR of 2-3. Incidence of Myocardial Infarction (MI) Dabiagatran • Displays a significantly greater risk for MI/Acute Coronary Syndromes (ACS). Rivaroxaban • Lower risk for MI/ACS When to discontinue Apixaban Rivaroxaban Dabigatran Cardioversion • Dabigatran has been shown to be an acceptable alternative to warfarin • No studies have evaluated rivaroxaban or apixaban Maybe a slide • whaT PERCENTAGE OF NEW RX FOR A FIB ARE NON WARFARIN DRUGS Summary