* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Genetic Disorders and Diseases
History of genetic engineering wikipedia , lookup
Behavioural genetics wikipedia , lookup
Tay–Sachs disease wikipedia , lookup
Gene expression programming wikipedia , lookup
Human genetic variation wikipedia , lookup
Gene therapy wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Quantitative trait locus wikipedia , lookup
Cell-free fetal DNA wikipedia , lookup
Genetic engineering wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Genetic testing wikipedia , lookup
Neuronal ceroid lipofuscinosis wikipedia , lookup
Microevolution wikipedia , lookup
Genome (book) wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Birth defect wikipedia , lookup
Fetal origins hypothesis wikipedia , lookup
Designer baby wikipedia , lookup
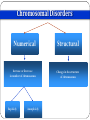
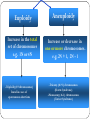
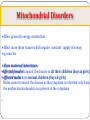
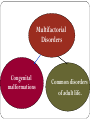
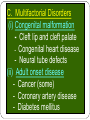
Genetic Disorders & Diseases Types & Inheritance Genetic Disorders & Diseases MUTATIONS (in the: Genome, Chromosome or Gene) GENETIC DISORDER in the form of decrease or increase in the amount of genetic material Or, abnormal genetic material LEADING TO Increase or decrease in the amount of gene products (proteins) OR, Defective function of the protein CLINICAL MANIFESTATIONS of GENETIC DISEASES Types of Genetic Diseases Some genetic disorders are entirely environmental and others are wholly genetic 1- Single Gene Disorders 2- Chromosomal Disorders 3- Multifactorial Disorders: used to describe the aetiology of the disorders 4- Mitochondrial Disorders 5- Acquired Somatic Genetic Diseases Chromosomal Disorders •Result from defect in: number (i.e. Numerical Disorders) of chromosomes OR structure (i.e. Structural Disorders) of chromosomes • Example: Trisomy 21 (Downs syndrome) • These disorders are quite common and affect about 1/800 live born infants • Account for almost half of all spontaneous first-trimester abortions • Do not follow Mendelian pattern of inheritance Chromosomal Disorders Numerical Structural Increase or Decrease in number of chromosomes Change in the structure of chromosomes Euploidy Aneuploidy Euploidy Increase in the total set of chromosomes e.g. 3N or 4N -Triploidy (69 chromosomes) found in cases of spontaneous abortions Aneuploidy Increase or decrease in one or more chromosomes. e.g. 2N + 1, 2N - 1 -Trisomy (46+1) chromosomes (Down Syndrome) -Monosomy (46-1) chromosomes (Turner Syndrome) Non-Disjunction Triploidy (69, XXY) Mitochondrial Disorders Effect generally energy metabolism. Effect more those tissues which require constant supply of energy e.g. muscles. Show maternal inheritance: Affected females transmit the disease to all their children (boys & girls) Affected males have normal children (boys & girls) Males cannot transmit the disease as the cytoplasm is inherited only from the mother & mitochondria are present in the cytoplasm • Multifactorial Disorders • Interaction between environmental & genetic factors. • Polygenic in nature, no single error in the genetic information. • Environmental factors play a significant role in precipitating the disorder in genetically susceptible individuals. • Tend to cluster in families • Do not show characteristic pedigree pattern of inheritance. • Examples: Obesity, DM, Hypercholesterolemia, Hypertension •Account for the majority of morbidity and mortality in developed countries Multifactorial Disorders Congenital malformations Common disorders of adult life. Single Gene Disorders • Caused by mutation in only one gene • Can lead to critical errors in the genetic information (lead to inborn errors of metabolism) • Follow Mendelian Inheritance • Occur at a variable frequency in different population • Over 7,000 single gene disorders have been identified. • May be - Autosomal - Sex-linked ⇒ Single Gene Disorders Inborn Errors of Metabolism Also called, Inherited metabolic diseases or congenital metabolic diseases. They are a large group of genetic disorders, resulting in metabolic defects due to a single gene mutation which leads to Reduced or absent gene product or Production of a different protein with abnormal function. ⇒ Single Gene Disorders ENZYME A C B ⇒ Single Gene Disorders General Effects of Inborn Errors of Metabolism Accumulation of a substrate or its metabolic derivatives that are harmful or may interfere with normal function of cells. Accumulation of metabolic pathways intermediates from alternative Decreased ability to synthesize essential compounds Defects in energy production ⇒ Single Gene Disorders Inheritance of inborn errors of metabolism Most of the inborn errors of metabolism are inherited as Autosomal recessive or X-linked disorders in nuclear DNA Few are inherited as autosomal dominant. Some may involve mitochondrial functions as they are linked to mt- DNA. The incidence of these diseases within different racial and ethnic groups varies with predominance of certain inborn errors of metabolism within particular groups. Some of these diseases occur in large numbers in communities in which consanguinity is common. ⇒ Single Gene Disorders ⇒ Inheritance of inborn errors of metabolism Inborn errors of metabolism are important causes of illness in children They may present as acute life-threatening illness in the neonatal period or become apparent later in childhood Rarely, clinical presentation is delayed until adult life. Inherited disorders may be detected in different stages during life Heterozygote carriers of a disease: It may be found during screening (as such performed on family members of a patient with muscular dystrophy). Before birth (intrauterine) Some inherited disorders can be detected before birth (as cystic fibrosis). Neonatal (newborn) screening (in first days of life) As for phenylketonuria In neonates (in first weeks of life) Many disorders involving single gene defects become apparent clinically (give symptoms & signs). Some disorders such as familial hypercholesterolemia may not be recognized until adult life. Before birth diagnosis of inherited metabolic diseases Prenatal diagnosis is important in detecting and preventing genetic diseases Prenatal screening of inherited metabolic disorders is done by demonstrating the metabolic defects by chorionic villus biopsy (in first trimester) & cultured fetal fibroblasts obtained by amniocentesis (in second trimester) Screening is indicated in: - Women with previously affected infant - Ethnic groups thought to have a high incidence of the carrier state -------------------------------------------------------------------------N.B. prenatal screening for chromosomal abnormalities is also performed (e.g. serum α- fetoprotein concentration for diagnosis of fetal neural tube defects & in Down’s syndrome) Techniques For Prenatal Diagnosis 1. Ultrasonography: It is safe and performed in the second trimester 2. Amniocentesis: Procedure risk : It has 0.5-1% of risk during procedure Performed in second trimester Widely available 3. Chorionic villus sampling Procedure risk : 1-2 % Performed in first trimester Specialized technique 4. Cordocentesis Procedure risk: 1% Performed in second trimester Specialized technique ⇒Techniques For Prenatal Diagnosis continued 5. Fetal tissue biobsy Procedure risk > 3% Performed in second trimester Very specialized technique Limited application General Criteria For Prenatal Diagnosis Criteria of the disorder to be diagnosed prenatally: 1. 2. 3. 4. 5. High risk Severe disorder Treatment not available Reliable prenatal test available Accepted to parents Before birth diagnosis of inherited metabolic diseases (cont.) 1- Prenatal Diagnosis (chrorinic villus biopsy) At earlier stage of pregnancy than amniocentesis: (from 10th week of pregnancy, first trimester) Biopsy can be used for karytyping and gene probe diagnosis. It takes ~ 10 days It has been applied particularly for Hemoglobinpathies (e.g. sickle cell disease, etc) cystic fibrosis Duchene's muscular dystrophy. Before birth diagnosis of inherited metabolic diseases (cont.) 2- Prenatal Diagnosis (amniocentesis) Carried out at about 15th week of pregnancy (second trimester) from amniotic fluid for cytogenic reasons (e.g. detect chromosomal disorders as Down’s syndrome) Diagnosis is usually made on the basis of enzyme studies carried out on fibroblasts cultured from the amniotic fluid. Screening of Newborns Programmes for screening all newborns for certain metabolic disorders are performed with the following criteria: 1- The disease should not be clinically apparent at the time of screening 2- The disease should have a relatively high incidence in the population screened. 3- The disease should be treatable & so results of screening test must be obtained before irreversible damage is likely to have occurred. 4- Screening test should be simple (qualitative) & reliable. Examples of screening programmes: Phenylketonuria Congenital hypothyroidism Galactossemia ⇒Screening of Newborns A positive screening qualitative test result should be confirmed by quantitative analysis In a second positive test, the individual might be required to be reassessed after a period of time in some cases. In Neonates & Infants Clinical features are often nonspecific and include failure to thrive, feeding difficulties, vomiting, hypotonia and fits, etc… There may be symptom-free period after birth It is very important to establish whether there is a relationship between onset of disease and changes in feeding patterns (e.g. galactosemia after introduction of milk). So what about role lab investigations in diagnosis?? Laboratory investigations for neonates & infants 1- Basic investigations: Carried as a part of normal care of a risk infant Nonspecific as determination of plasma glucose & blood gases May show results consistent with inherited metabolic disease. 2- Metabolic tests: Provide an additional evidence The measurement of specific metabolites Determination of enzyme activity (of the enzyme responsible for the inherited metabolic disorder). May be a part neonatal screening i.e. for phenylketonuria in first days of life 3- Sequencing of the specific gene (molecular genetic techniques): Using PCR and gene sequencer, etc….(Molecular Genetics) Karytyping may help in some cases: (chromosomal study, cytogenetics) Examples of Genetic Diseases A.Single-gene Disorders - Adenosine deaminase deficiency - Alpha-1-antitypsin deficiency - Cystic fibrosis - Duchenne muscular dystrophy - Familial hypercholesterolemia - Hemophilia A and B - G-6-PD deficiency - Phenylketonuria - Sickle cell anaemia - Thalassaemia C. Multifactorial Disorders (i) Congenital malformation - Cleft lip and cleft palate - Congenital heart disease - Neural tube defects (ii) Adult onset disease - Cancer (some) - Coronary artery disease - Diabetes mellitus Examples of Multifactorial disorder Disorder Incidence Cleft lip/ Cleft palate 1/250 – 1/600 Congenital heart disease Neural tube defects 1/125 – 1/250 1/100 – 1/500 Coronary heart disease 1/15 – Variable Diabetes mellitus 1/10 – 1/20 Cancer variable D.Mitochondrial Disorders Lebers hereditary optic neuropathy E. Acquired somatic genetic disorders Some forms of cancer