* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Wheat Amylase Trypsin Inhibitors as Divers of Innate Immunity in
Plant disease resistance wikipedia , lookup
Behçet's disease wikipedia , lookup
Molecular mimicry wikipedia , lookup
Vaccination wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Globalization and disease wikipedia , lookup
Immune system wikipedia , lookup
Germ theory of disease wikipedia , lookup
Sjögren syndrome wikipedia , lookup
Inflammatory bowel disease wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Social immunity wikipedia , lookup
Adaptive immune system wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Herd immunity wikipedia , lookup
Innate immune system wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
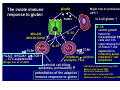
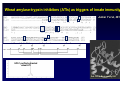
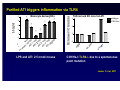
Wheat Amylase Trypsin Inhibitors as Divers of Innate Immunity in Celiac Disease Detlef Schuppan Professor of Molecular and Translational Medicine, Dept. Medicine I, Univ. of Mainz, Germany Professor of Medicine, Division of Gastroenterology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, USA Associazione Italiana Celiachia, Florence, March 29-31, 2012 HARVARD MEDICAL SCHOOL Hallmarks of celiac disease • Dietary gluten from wheat, barley, rye as trigger • Genetic Predisposition (HLA-DQ2 or – DQ8) • IgA autoantibodies to endomysium and reticulin Definition of non-celiac gluten sensitivity Symptoms induced by gluten ingestion in the absence of 1-3 of the 3 defining hallmarks of (adapative imunity )of celiac disease GS patients are orphans living in a (diagnostic and therapeutic) no man‘s land „gluten sensitivity“ without DQ2/8 and celiac auto-Abs ? Verdu EF et al, Am J Gastroenterol 2009 Role of the Innate Immune System in celiac disease – prior work • Stimulation of biopsies from CD patients with whole gliadin digests or α2 gliadin p31-43 increases the number of IL-15 positive cells within the lamina propria (Maiuri et al, Lancet 2003) • p31-43 induces MICA on intestinal epithelial cells via IL-15, providing a target for cytotoxic IELs (Hue et al, Immunity 2004) • Peptic-tryptic gliadin digests and certain gliadin peptides induce activation and maturation of monocytes, macrophages and dendritic cells (Tuckova et al, J Leuk Biol 2002; Palova-Jelinkova et al, FEBS Lett 2004; Nikulina et al, J Immunol 2004; Palova-Jelinkova et al. J Immunol., 2005; Cinova et al, J Clin Immunol 2007; Rakhimova et al., J Clin Immunol 2008) • Gliadin increases intestinal permeability and induces DC activation via MyD88 (implication of CXCR3 on intestinal epithelial cells as gliadin receptor) (Thomas et al., J Immunol., 2006; Lammert et al, Gastroenterology 2008) Problems: 1. LPS contamination not strictly ruled out 2. No reproducible identification of a certain (set of) gliadin peptide(s) 3. No receptor identified The innate immune response to gluten gliadin PAMPs Major role of professional APC ? Is it all gluten ? IL -15: IL-15: MIC-A/B NKG2D (CD94) NK -like NK-like TCR+ HLA-E NKG2A+ TCR+ → CTL suppression Bhagat G et al, JCI 2008 IL-15 • central growth IL-15 factor for intraepithelial NK cells and CTL • major driving force of clonal T cell CTL TCR+ expansion in CD8+ refractory sprue & interferon , perforin intestinal T cell granzyme, FasL lymphoma epithelial cell killing, apoptosis, permeability potentiation of the adaptive immune response to gluten Jabri B et al, Gastroenterology 2000 Maiuri L et al, Lancet 2003 Hue S et al, Immunity 2004 Meresse B et al, Immunity 2004 Rakhimova M et al, J Clin Immunol 2008 Wheat amylase-trypsin inhibitors (ATIs) as triggers of innate immunity Junker Y et al, 2012 HPLC purified extracted wheat ATI Oda Y et al, Biochemistry 1997 Purified ATI triggers inflammation via TLR4 5 * Monocyte derived DCs * 4 3 * * * 3 TLR4 wt and KO mice fed ATI wildtype C3H/HeJ 2 2 * * 1 1 0 AT I5 0 g 0 LPS and ATI: 2.5 nmol/ mouse C3H/HeJ: TLR4-/- due to a spontaneous point mutation Junker Y et al, 2011 Characteristics and functions of cereal ATIs • Mr 12-16 kDa • 5 (4) highly conserved S-S bonds • Conserved 4 helix conformation • Hydrophobic interaction sites • Forming mono - tetramers • Highly resistant to (intestinal) proteolysis Tatham and Shewry, Clin Exp Allergy 2008 Characteristics and functions of cereal ATIs (2) • Up to 11 homologues in wheat (CM1-3, 16, 17, 0.19, 0.28, 0.53……) • Tetraploid encodes fewer than hxaploid wheat (lack of the D chromosome: CM1, 3b, 7, 0.19, 0.28) • Pest control (inhibition of parasite amylase and trypsin like activities) • Major wheat allergens (baker‘s asthma), food allergy to wheat and barley) Tatham and Shewry, Clin Exp Allergy 2008 Allergen as TLR4 agonist 2nd allergen MD2 TLR4 Derp1…… MD2-mimetics: Derp2, ATI ? Derp1 Derp2 from house dust mite ATI ? IRF3 CD23+ IgE Allergy IL-4, IL-13 IL-6 Rantes MCP-1 IL-8 Th2 TLR4 mediated potentiation of Th1 or allergic responses NFkB TNFα, IL-12, IL-15 Th1 modified from Trompette A et al, Nature 2009 Wills-Karp M et al, Mucosal Immunol 2010 Origin of Wheat Increase of content of immunogenic epitopes and ATI with higher ploidity Celiac disease Cd is the best defined and most frequent intestinal (auto -) (auto-) immune disease disease,, with gluten (and ATI) as triggers triggers,, HLA HLA-DQ2 (DQ8) as necessary genetic predisposition and TG2 as patho -genetically linked autoantigen patho-genetically Most cases are not associated with diarrhea or overt malabsorption malabsorption,, but are are silent or atypical Disease severity depends depends on on 1. 1. gluten gluten dose, 2. HLA HLA-DQ2(DQ8) -gene dose, 3. innate immunity ((triggered triggered by DQ2(DQ8)-gene ATIs ), 44.. additional polygenetic and environmental factors ATIs), Innate immunity to cereal ATI likely impacts other intestinal inflammatory diseases