* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lecture Notes # 3
Density functional theory wikipedia , lookup
Tight binding wikipedia , lookup
Identical particles wikipedia , lookup
Interpretations of quantum mechanics wikipedia , lookup
Erwin Schrödinger wikipedia , lookup
Renormalization group wikipedia , lookup
Double-slit experiment wikipedia , lookup
History of quantum field theory wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Renormalization wikipedia , lookup
Coherent states wikipedia , lookup
Atomic theory wikipedia , lookup
EPR paradox wikipedia , lookup
Path integral formulation wikipedia , lookup
Hidden variable theory wikipedia , lookup
Wave function wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Schrödinger equation wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Quantum state wikipedia , lookup
Dirac equation wikipedia , lookup
Density matrix wikipedia , lookup
Probability amplitude wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
Canonical quantization wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Atomic orbital wikipedia , lookup
Electron configuration wikipedia , lookup
Electron scattering wikipedia , lookup
Hydrogen atom wikipedia , lookup
Wave–particle duality wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Matter wave wikipedia , lookup
Particle in a box wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
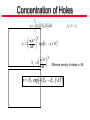
Lecture Notes # 3 • Understanding Density of States – Solve 1-D Schrödinger equation for particlein-a-box – Extend to 3-D – Invoke periodicity requirement – Solve for density of states Review of Quantum Mechanics H H Hamiltonia n operator Mathematic wavefunct ion depicting nature of electron in space/time Energy or allowable quantized energies of that electron • Often times you do not know or , but you have boundary conditions and want to solve for possible values of and a functional form of Review of Quantum Mechanics • Most general case: Time independent 2 2 H U x, y , z 2m Kinetic E. Potential E. • How do we know first part is K.E? 1 2 mv classicall y 2 1 2 p2 mv 2 2m pˆ i is quantum momentum operator KE 1 2 2 2 So, mv 2 2m Review of Quantum Mechanics For r exp ik r pˆ a Momentum operator Eigenvalue pˆ i r i r iik r k r r a = Momentum Eigenvalue p mv k k v velocity of particle m Particle in a 1-D Box • Free electron floating around in vacuum • Let’s impose some boundary – confine it to a region of space, a box or a unit cell in 1-D U Confine it by setting U outside the box and U = 0 inside the box U Confined e- U=0 0 L x • Since U(x) = 0 for 0<x<L, we can drop U(x) out of the Hamiltonian, which becomes 2 2 H 2m Particle in a 1-D Box • Because U outside the box, we know the eCANNOT be there, so we get the boundary condition: x 0 x L 0 Because the Hamiltonia n must give back an eigenvalue , we must pick f x where f x Af x and f 0 f L 0 • We do not care what happens between 0 and L, so the simplest solution is just: n A sin x , n 1,2,3,4... L for x 0, sin( 0) 0 OK for x L, sin( nL) 0 OK Particle in a 1-D Box • Plug into the Schrödinger equation to make sure H = E 2 d 2 n A sin 2 2m dx L x 2 d n n A cos 2m dx L L 2 2m x n 2 2 n x 2 A sin L L 1 n n A sin 2m L L 2 Energy, E x Particle in a 1-D Box • Energy values are quantized since n is an integer • n=1 is lowest energy state, n=2 has higher energy, etc. n=4 E n=3 9x n=2 4x n=1 18 0 16 14 L • We can map out (x,n) vs. E 12 10 n E x 2 2mL 2 2 2 8 • Allowed energy states 6 4 2 0 0 1 2 n 3 4 Particle in a 1-D Box • Now let’s fill up the states with electrons. Suppose we have N e- we want to pour into our 1-D box. • For N e- you can calculate the energies since we know we can have 2e-/n states (two spins). • So N electrons fills nF= N/2 states. • The highest energy state, nF, gives F, the Fermi energy. 2 F nF 2m L Fermi-Dirac Distribution • Fermi energy is well defined at T = 0 K because there is no n E x thermal promotion 2mL • At high T, there is F thermalization, so F is not as clear 2 2 18 16 14 2 12 2 10 8 6 4 2 0 0 1 2 n 3 • Officially defined as the energy where the probability of finding an electron is ½ • This definition comes from the Fermi-Dirac Distribution: 1 f exp / k BT 1 • This is the probability that an orbital (at a given energy) will be filled with an e- at a given temperature • At T=0, =F and = F, so f(F)=1/2 4 Particle in a 3-D Box • Let’s confine e- now to a 3-D box • Similar to a unit cell, but e- is confined inside by U outside the box • Schrödinger’s equation is now 2 2 2 2 2 2 2 x, y, z x, y, z 2m x y z • You can show that the answer is: nx n y n x, y, z A sin x sin L L nz y sin z L • We now have 3 quantum numbers nx, ny, and nz that are totally independent • (1,2,1) is energetically degenerate with (2,1,1) and (1,1,2) Particle in a 3-D Box • Now let’s repeat this box infinitely in each direction to get a repeated “unit cell” • What’s different about this situation? – U(x,y,z)=0 – No region where U = infinity • So, there’s really no reason that x 0 x L 0 since U x 0 U x L • We don’t need those boundary conditions anymore Periodic Boundary Condition • For now, we don’t need such a strict boundary condition • Make sure is periodic with L, which would make each 3D box identical • Because of this, we’ll have a periodic boundary condition such that x L, y, z x, y, z • Wave functions that satisfy this periodic B.C. and are solutions to the Schrödinger equation are TRAVELING WAVES (not a standing wave anymore) Periodic Boundary Condition • Bloch function k r exp ik r • Wave vector k satisfies 2 4 k x 0; ; ;... L L • Etc. for ky and kz • Quantum numbers are components of k of the form 2n/L where n=+ or - integer exp ik x x L exp i 2nx L / L exp i 2nx / Lexp i 2n exp i 2nx / L exp ik x x • Periodicity satisfied Back to Schrödinger Equation • Substitute k r exp ik r into 2 2 2 2 2 2 2 k r k k r 2m x y z gives 2 2 2 2 k k k x k y2 k z2 2m 2m • Important that kx can equal ky can equal kz or NOT p̂ i • The linear momentum operator for k r exp ik r pˆ k r i k r k k r so the plane wave k r is an eigenfunct ion of linear momentum with an eigenvalue of k , and the particle velocity in orbital k is k v m Fermi Level in 3-D kx • Similarly, can calculate a Fermi level F Fermi level 2 2 kF 2m kF kz Vector in 3-D space • Inside sphere k<kF, so orbitals are filled. k k>kF, orbitals are empty • Quantization of k in each direction leads to discrete states within the sphere • Satisfy the periodic boundary conditions at ± 2/L along one direction This is a sphere only if • There is 1 allowed wave vector k, with NOTE: k =k =k . Otherwise, we have an ellipsoid and have to distinct kx, ky, kz quantum #s for the recalculate everything. That can 3 volume element (2/L) in k-space be a mess. Sphere: GaAs (CB&VB), Si (VB) • So, sphere has a k-space volume of Ellipsoid: Si (CB) y x 4 V k F3 3 y z Number of Quantum States • Number of quantum states is 4 k 3 F total volume 3 3 volume of 1 allowed quantized state 2 L • Since there are 2 e- per quantum state 4 k 3 3 F L3 3 N 2 2 kF 3 2 3 L V N 2 k F3 3 Solve for k F , 3 N k F V 2 1 3 • Depends on e- concentration Density of States • Plug kF into 2 2 F k 2m 3 N F 2m V 2 2 2 3 • Relates Fermi energy to electron concentration • Total number of electrons, N: 3 V 2m 2 N 2 2 3 • Density of states is the number of3 orbitals per unit energy dN V 2m 2 1 2 D 2 2 d 2 Relate to the surface of the sphere. For the next incremental growth in the sphere, how many states are in that additional space? Density of States • Divide by V to get N/V which is electron density (#/cm3) • Volume density of orbitals/unit energy for free electron gas in periodic potential 3 1 2m 2 1 2 D 2 2 2 Starting point energy Effective mass of e- 1 2m D 2 2 2 * e 3 2 E ECB 2 1 CB Effective mass of h+ 1 2m 2 D 2 2 * h 3 2 EVB E 2 1 Start from VB and go down VB Concentration of Electrons n D E f E dE fun e e EC “EF” 3 m kT 2 exp EC / kT n 2 2 2 * e m kT N C 2 2 2 * e 3 2 Effective density of states in CB n NC exp EC EF / kT Writing it with a minus sign indicates that as E difference between EC and EF gets bigger, probability gets lower Concentration of Holes p EV D E f E dE h h fh 1 fe 3 m kT 2 exp EV / kT p 2 2 2 * h m kT NV 2 2 2 * h 3 2 Effective density of states in VB p NV exp EF EV / kT Intrinsic Carrier Concentration EC EF EF EV n p N C NV exp kT EC EV n p N C NV exp kT EC EV EG , the band gap n p NC NV exp EG / kT Entropy term Enthalpy term n p ni2 ni intrinsic carrier concentrat ion Constant for a given temperature. Intrinsic = undoped Intrinsic Carrier Concentration EG ni N C NV exp 2 kT ni constant for a given T means that n p is constant, too, which holds under equilibriu m and away from it • • • • ni Ge: 2.4 x 1013 cm-3 Si: 1.05 x 1010 cm-3 GaAs: 2 X 106 cm-3 At 300 K At temperature T, n = p by conservation Add a field and np = constant, but n does not equal p As n increases, p decreases, and vice versa Useful to define Ei, which is Ei = EF when it is an intrinsic semiconductor (undoped), so n = p = ni EC Ei Ei EV ni N C exp NV exp kT kT Intrinsic Fermi Level EC Ei Ei EV ni N C exp NV exp kT kT NV EC Ei Ei EV ln kT kT NC NV Ei EC EV Ei kT ln NC NV 2 Ei EV EC kT ln NC NV 2Ei EV EC EV kT ln NC EG kT NV Ei EV ln 2 2 NC Intrinsic Fermi Level EG kT NV Ei EV ln 2 2 NC • This says that the intrinsic Fermi level (relative to the valence band) is about mid-gap ± the (kT/2)ln(NV/NC) scaling factor kT NV ln 2 NC EG (eV) -13 meV for Si 1.12 35 meV for GaAS 1.42 0.67 - 7 meV for Ge • So Ei for Si and Ge is slightly below mid-gap. Ei for GaAs is slightly above. It is minor compared to EG, but just so you know • All of this has been intrinsic with no dopants Dopants • Let’s consider adding dopants n-type carriers n ni N D ED ECB Ei EVB Depends on EG, T Depends on D, T, and dopant density D ECB ED At T=0, all donor states are filled. Hence, n = 0. But at room temperature in P doped Si, 99.96% of donor states are ionized. At mid temperature, if N D ni , then n N D At high temperature, such that ni N D , then n ni Dopants n p ni2 still holds, just substitute n ni N D ni2 p at room temperatu re, n N D for typica l dopant densities ni N D Say N D 1016 cm 3 N D 1016 cm 3 n 1016 cm 3 n NC exp EC EF / kT n 1016 cm 3 19 3 exp EC EF / kT N C 10 cm about 60 mV/decade 3 decades x 60 mV 180 mV 180 meV down