* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Interventional cardiac catheterization
Survey
Document related concepts
Management of acute coronary syndrome wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Coronary artery disease wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Drug-eluting stent wikipedia , lookup
Cardiac surgery wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Aortic stenosis wikipedia , lookup
Atrial septal defect wikipedia , lookup
History of invasive and interventional cardiology wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
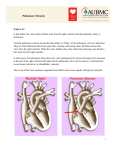
Dextro-Transposition of the great arteries wikipedia , lookup
Transcript
1 INTERVENTIONAL CARDIAC CATHETERIZATION Jaana Pihkala M.D., David Nykanen M.D., FRCP(C), Robert Freedom M.D., FRCP(C), FACC, Lee N. Benson M.D., FRCP(C), FSCAI, FACC From the Division of Cardiology, The Hospital for Sick Children, The University of Toronto School of Medicine, Toronto, Ontario, Canada SYNOPSIS Over the past decade, the focus of the pediatric catheterization laboratory has changed dramatically from its primary diagnostic function to a conduit for therapeutics. Today, therapeutic catheterization techniques have replaced conventional surgery for many lesions. The percutaneous transcatheter procedures may be broadly grouped as dilations (septostomy, valvuloplasty, angioplasty, and endovascular stenting) or as closures (vascular embolization and device closure of defects). New methods, tested through research protocols, combined with continuous critical evaluation of established techniques continue to ensure improved and sustained results. Introduction Over the past decade, the focus of the pediatric catheterization laboratory has changed dramatically from its primary diagnostic function to a conduit for therapeutics. Although the first application of such catheter interventions was described over 44 years ago by Rubio-Alvarez (145), it was not until 1966, when Rashkind and Miller (132) described the technique for percutaneous creation of an atrial septal defect in the setting of complete transposition of the great arteries, did such catheter maneuvers decisively impact patient management (82,134-136). Today, therapeutic catheterization techniques have replaced conventional surgery for many lesions. New methods, tested through research protocols in specialized centers, combined with continuous critical evaluation of established techniques continue to ensure improved and sustained results. The primary consideration of the practicing pediatric cardiologist should not be whether it is possible to perform such techniques, but whether their clinical utility, morbidity and mortality justifies a non-surgical approach for their patient. Unsuccessful outcomes, including complications and nondefinitive outcomes, may in part yield to experience and improvements in technique. 2 Therapeutic interventions, like cardiac surgery, have three principal objectives: improvement and/or preservation of cardiac function, improvement in longevity, and maintenance or improvement in the quality of life. Catheterization interventions may be corrective, reparative or palliative. Catheter therapies may also be applied as adjuncts to surgery. There has developed cooperation between the pediatric cardiologist and pediatric cardiac surgeon in planning staged repairs of complex congenital heart disease. This type of cooperation will increase further and is already contributing to a far better outcomes for many complex congenital heart lesions. Dilation of Valvular Lesions Typical valvular stenosis is characterized by fused, partially absent, or underdeveloped commissures with a small eccentric orifice. The technique used to relieve congenital or acquired stenotic valvar lesions is well established. The valve morphology, size, and exact position are visualized angiographically. Accurate determination of the valve annulus diameter is performed using calibrated marker catheters or computerized angiographic systems. The dilating balloon catheter is positioned over a wire across the site of stenosis and the balloon inflated several times to ensure that the indentation or “waist” of the stenotic region disappears. Echocardiographic (12), intraoperative (11,77,177), and postmortem (98) examinations have documented commissural or pericommissural splitting as the mechanism of stenosis relief, often with complete separation of the previously fused leaflets. Appropriate choice of balloon size, in terms of both balloon diameter and length, remains critical in achieving good results and preventing complications. The use of longer balloons facilitates proper positioning and maintaining position across the valve during balloon inflation. A double balloon technique using catheters with smaller balloon profiles can be employed to avoid reduction in systemic blood pressure and heart rate during balloon inflation (temporary iatrogenic outflow tract obstruction) and to reduce the vessel trauma caused by the larger diameter catheters. Dilation of the Pulmonary Valve Since first reported in 1982 (67), percutaneous balloon pulmonary valvuloplasty has replaced surgery as the treatment of choice for patients of all ages with moderate to severe pulmonary valve stenosis. The indications for balloon therapy are the same as those for surgical valvotomy: a transvalvular echocardiographically determined gradient of ≥50 mmHg with normal cardiac output (127). In critical pulmonary valve stenosis, the pulmonary valve pressure gradient may be significantly higher or lower than 50 mmHg, depending on cardiac output, right ventricular function, and patency of the arterial duct. 3 The diameter of the balloon used for valvuloplasty ranges between 1.2 to 1.4 times that of the pulmonary valve annulus (123,124,126). Complications are rare, pulmonary regurgitation may occur in some patients but is typically mild and inconsequential (162). The best results have been achieved in patients with typical pulmonary valve stenosis manifest with thickened doming leaflets, fusion of the commissures and a normal sized annulus. Patients with dysplasia of the pulmonary valve leaflets have also undergone dilation with varying degrees of success (26,86,108), those with significant annular hypoplasia, short main pulmonary artery, and supravalvar narrowing, have less effective procedures. Balloon dilation is not useful for treatment of fixed infundibular, subpulmonary outflow tract stenosis unassociated with pulmonary valve stenosis (2), although dynamic infundibular obstruction will resolve with valve dilation. Long-term outcomes after treatment of pulmonary valve stenosis is influenced by residual pressure loading of the right ventricle from residual or recurrent valve obstruction, and volume loading as a result of pulmonary insufficiency. Balloon dilation appears to provide long-term gradient relief in the majority of patients, with regression of subpulmonary muscular obstruction (36,90,111,143). In a series of 533 patients reported from the 22 institutions contributing to the Valvuloplasty and Angioplasty of Congenital Anomalies (VACA) Registry, acute hemodynamic success occurred in 74% of patients (successful procedures defined as gradients of <36 mmHg) (95). In a follow-up study from the Registry, an average of 3 years after the procedure, 23% of the patients had an outcome judged to be suboptimal due to either a residual gradient of >36 mmHg or further treatment of pulmonary stenosis requiring either repeat balloon valvuloplasty or valve surgery. Significant predictors of suboptimal long-term outcomes included small valve hinge point diameters and a higher gradient immediately after the procedure (95). Valvuloplasty in the neonate with critical pulmonary valve stenosis has also been shown to be effective (21,45). Intermediate term follow-up has demonstrated sustained hemodynamic relief in the majority of patients (166). After balloon pulmonary valvuloplasty in infants with either critical or severe pulmonary valve stenosis, right heart structures increase in size at a rate that parallels or exceeds the rate of somatic growth (74,166,174). However, acute complication rates have been reported from 10% to 30% from early application of this technique in the most young (22,30,47,166). Serious complications including death, stroke, tamponade, and necrotizing enterocolitis have occurred in approximately 10 % of the patients. The incidence of major and minor complications was inversely related to age (162), but with increased experience, complications from the procedure are today, rare. 4 Recently, modified catheterization techniques have been developed for treatment of newborns with pulmonary atresia and intact ventricular septum. The atretic membranous valve can be perforated with a wire or with a hot tipped catheter using laser (43) or radiofrequency (47,66) energy. Once perforated, the valve is balloon dilated to create unobstructed continuity between the right ventricle and pulmonary artery. Additionally, in patients with tetralogy of Fallot and some additional forms of cyanotic heart disease, balloon pulmonary valvuloplasty has been shown to improve oxygenation, growth of the pulmonary arteries, and right ventricular outflow tract avoiding the need of a systemic-pulmonary shunt as intermediate palliation (92,118,125,161,163). Dilation of the Aortic Valve Aortic stenosis can lead to ventricular failure with subendocardial ischemia. Severe stenosis is associated with a 19% risk of sudden death, and the lesion is often progressive (175). The transaortic pressure gradient and left ventricular peak systolic pressure can be reduced with balloon valvuloplasty, and the improvement has been shown to persist in the majority of patients (29,96). The indications for balloon valvuloplasty are generally the same as those used for surgical valvotomy, namely, peak-to-peak gradients of ≥70 mmHg irrespective of symptoms or gradients of ≥50 mmHg with associated symptoms, ST-T wave changes on the electrocardiogram indicative of myocardial ischemia at rest or with exercise (131). However, patients who have significant aortic valve regurgitation are not considered candidates for balloon valvuloplasty. The difficulties associated with the procedure include the passage of the wire across the valve, maintaining the position of the balloon across the valve during inflation, a significant drop in blood pressure and heart rate during inflation, and the possible damage to the femoral arteries caused by the large dilation catheters. Many of these issues have been addressed with stabilizing wires, longer balloons with low profiles, and a variety of ingenious methods to cross the aortic valve. The size of the balloon used should not be larger than that of the aortic valve annulus, as balloons larger than the valve annulus increase the risk of aortic regurgitation (37,152). The success rate for balloon valvuloplasty for congenital aortic stenosis has been 87% to 97% (38,103,142,147), with an average gradient relief of 55% to 70% (76,103). Complications are relatively rare although their potential, such as arterial occlusion (64) (especially in young children), and aortic regurgitation should be recognized (53,64). Yet, no significant differences in mortality, morbidity, or need for reintervention within 1 year have been noted between patients treated with surgical valvulotomy and those treated with balloon valvuloplasty (38,64). From several studies, 75% of the patients have been free of repeat intervention 4 5 years after dilation, and 50% of the patients remain free of repeat intervention at 8 years follow-up (53,103). Aortic stenosis in the neonatal period has been a more difficult problem, with a high initial mortality. With balloon valvuloplasty, hemodynamic and clinical improvement can also be achieved in the neonate (29,69,152). Recent studies of balloon valvuloplasty have shown it to be as effective and safe as surgical transventricular dilation in the treatment of critical aortic stenosis in newborn, with an early mortality of 11% (106). Late survival and functional class are excellent for patients surviving the initial hospitalization, but most require further intervention within 10 years (39). Because of the small size of the femoral arteries, arterial occlusion rate is high and use of antegrade (85), umbilical (9) or carotid (34,46,84) approaches have been developed to avoid this complication. With improved catheter technologies, mortality from the procedure is rare and morbidity now low (85). Relief of left ventricular outflow tract obstruction has also been achieved with balloon dilation in discrete subaortic stenosis in both children and adults (32). Left ventricular outflow tract obstruction caused by hypertrophic cardiomyopathy or a fibromuscular tunnel appears not to be dilatable with a balloon, and they require surgical resection. Balloon dilation of supravalvar aortic stenosis has not been considered to be an appropriate application of the technique due to potential damage to the aortic root and compromise to the coronary artery ostium (2). Dilation of the Mitral Valve Congenital mitral stenosis is a very rare condition. It can be isolated or more often associated with other defects, such as a patent arterial duct, aortic stenosis, coarctation of the aorta, hypoplasia of the left ventricle, and papillary muscle anomalies (23). Disease severe enough to require intensive medical management or intervention during infancy is rare. Despite contemporary medical and surgical therapies, symptomatic infants continue to have high morbidity and mortality rates. Balloon dilation of congenital mitral stenosis can provide symptomatic relief in many, allowing postponement of valve replacement, although infants with a supravalvular mitral ring do better with surgery (48,101,159). Generally, a double balloon technique is used. A number of complications have occurred, including left ventricular perforation, complete atrioventricular block, mitral valve leaflet damage, and severe mitral regurgitation (101). The efficacy of balloon dilation for congenital mitral stenosis continues to be evaluated. Rheumatic mitral valve stenosis results from fibrosis of the mitral ring, commissural adhesion, and contracture of the valve leaflets, chordae, and papillary muscles. Experience with balloon dilation of 6 rheumatic mitral valve stenosis has been widespread and considerably more successful than that with congenital valve stenosis. Successful reduction of transvalvular mitral gradients has been reported in both pediatric and adult populations (63,109,153). Balloon dilation valvuloplasty is now an acceptable alternative to surgical treatment if not treatment of choice for rheumatic mitral valve stenosis. The indications for balloon therapy are symptoms associated with moderate to severe obstruction (mitral valve area <1.5 cm²) without significant mitral regurgitation and the lack of any demonstrable left atrial thrombus. Dilation of Bioprosthetic Valves Bioprosthetic valve-containing conduits are frequently used in surgical corrections in various forms of congenital heart disease, e.g., the Rastelli repair for ventriculoarterial discordance, truncus arteriosus and various forms of right ventricular outflow tract atresias. Unfortunately, these conduits have a high failure rate in children and adolescents of >40% at 3 to 5 years (51,72,100) after implantation. Degenerative changes leading to obstruction of the bioprosthetic valve leaflets have been observed in all types of valved conduits (176). Such calcific degeneration appears to occur more frequently and more rapidly in younger children than in older children and adults. Valve failure is characterized by calcification of the commissures and within leaflets, often with leaflet tears and ulcerations (149), as well as the development of an obstructive endoluminal peel in valve containing conduits. In addition, there may be stenosis at the anastomotic sites of the conduit to the pulmonary arteries, or, within the extension of the conduit to the anterior wall of the right ventricle. Case reports of balloon dilation of such valves have noted an average 40% gradient reduction with increased valve incompetence in the majority of the patients (176). The largest experience is with balloon dilatation of bioprostheses in pulmonary position. Although the results are not uniformly successful, significant gradient reduction along with either avoidance or postponement of replacement of the prosthetic valve conduit have been documented (see Endovascular Stents). Similar favorable results have been reported for bioprosthetic valves in tricuspid, mitral and aortic positions (131). However, dilation of left-sided bioprosthetic valves can carry significantly more risk for complications (72). Vascular Angioplasties Vascular stenoses can occur in isolation or as a component of several well described syndromes (such as Noonan’s or Williams’ syndromes or congenital rubella infection), or as an acquired postoperative 7 lesion (148,160). Histologically, vascular stenoses present a spectrum from normal to exaggerated internal or medial hyperplasia with alterations in the proportions of elastin, collagen, and smooth muscle (5). The lesions acquired after surgery have in addition mural and perivascular fibrosis (15,28). The technique of balloon angioplasty resembles that of balloon valve dilation utilizing the same catheter, with a cylindrical, fixed maximal diameter. The balloon size is determined from the size of the vessel proximal and distal to the stenosis, as well as the diameter of the narrowing. The balloon catheter is passed over a guide wire and positioned across the area of the stenosis and inflated with relatively high intra-balloon pressures. This stretches the area of stenosis to the predetermined diameter of the balloon. Balloon diameters are increased until a waist is seen, frequently multiple, serial waists are noted along the balloon length with inflation (141). The inflation is repeated several times until the waist(s) in the balloon disappears. Because of the risk of vessel rupture, care is taken not to manipulate a guide wire or a catheter over the area of a freshly dilated vessel. Successful angioplasty results in longitudinal or oblique, intimal or medial tears (15,28) with organization of intramural hemorrhage and scar formation as the mode of healing. Neoendothelialization is complete in 3 to 6 weeks while areas of mural thinning within the scar may predispose to aneurysm formation (15). According to a recent statement by the American Heart Association (2), balloon angioplasty is appropriate therapy for recoarctation of the aorta, systemic vein stenosis and pulmonary artery stenosis in the pediatric age group. Additionally, angioplasty may be indicated in native coarctation of the aorta in patients older than 6 months, systemic-to-pulmonary artery shunts, and the patent arterial duct. Balloon angioplasty of pulmonary vein stenosis, on the other hand, whether congenital or surgically acquired, has had disappointing results, either acutely unsuccessful or developing early recurrent stenosis (2). Recently, balloon dilation of vascular stenoses with stent implantation has shown promising results (see Endovascular Stents). Dilation of Coarctation of the Aorta Coarctation of the aorta (CoA) is the third most common congenital malformation of the cardiovascular system, occurring in 4.1/10 000 newborns, and in approximately 6% to 8% of patients with congenital heart disease. Surgery has been the standard therapy for CoA for 50 years, but the operation is associated with a clear morbidity and mortality. The incidence of reoperation (10% to 30%) in patients operated on in early infancy is significantly higher than in those repaired beyond 1 year of age (7). Surgery for recurrent coarctation is technically difficult and has an associated increased morbidity and mortality (6,165). 8 Percutaneous balloon angioplasty is the treatment of choice in recoarctation (reCoA) after surgical repair, irrespective of surgical technique. In several large studies with a long follow-up (up to 12 years), the immediate success rate has been reported to range between 65% to 100%. The rate of recurrent stenosis after balloon angioplasty of reCoA has been in the range of 30% (3,24,54,55,97,128,180,181). Procedure related mortality has been low, from none to 2.5% (54). Aortic arch hypoplasia seems to be a strong predictor of poor acute hemodynamic outcome, or recurrence (3,181). Balloon angioplasty for a previously unoperated (native) CoA is controversial. In the neonate, the rate of restenosis after balloon dilation is high and dilation should be considered as palliative. The restenosis rate gradually decreases during the first year of life (117). Balloon dilation of native CoA is more widely applied in the older child. It has been found to be a safe procedure, with good early hemodynamic results, and restenosis rates between 7% and 12% from large studies (8,35,172). In the summary data of the VACA Registry, including 907 procedures (422 CoA and 548 reCoA) performed in 907 patients from 25 centers (97), the acute success rate was 81% for native CoA and 75% for reCoA. Complications of balloon angioplasty of native CoA were equivalent or slightly superior to those of recurrent aortic obstructions. The procedure related mortality was 0.7% in both groups. The main issue challenging balloon angioplasty in CoA is the possibility that weakening the aortic wall will eventually lead to aneurysm formation. After balloon angioplasty, the rate of aneurysm formation has varied from none to 14% for reCoA (3,24,54,180,181), and from 2% to 6% for native CoA (35,99). However, aneurysms have also been found after surgical prosthetic patch angioplasty (in 5% to 21% after 10 years) and subclavian aortoplasty. Cystic medial necrosis within the coarcted segment and the development of aneurysms suggest an intrinsic substrate for their formation but this has not been substantiated in all reports (57,62). Other complications after balloon angioplasty have included femoral artery trauma (18), neurological events, and postcoarctectomy syndrome (3,24,54,180). After the procedure, careful follow-up is recommended with chest radiographs, echocardiograms, and magnetic resonance imaging to detect aneurysm formation and reCoA. The indications for balloon dilation of CoA are essentially the same as those for surgery, resting hypertension proximal to the CoA with a resting systolic blood pressure gradient across the narrowed segment ≥20 mmHg or angiographically severe CoA with extensive collaterals. The indications for balloon dilation of native CoA and for reintervention for reCoA after balloon angioplasty or surgery are the same. Arch morphology is an important factor in the choice of treatment, regardless of age. A discrete CoA with otherwise near normal aortic dimensions is an ideal candidate for balloon dilation. 9 Patients with long tubular lesions should be referred to surgery. Transcatheter stent implantation may have a role in selected patients with CoA, as discussed later in this chapter. Dilation of Pulmonary Artery Stenosis Causes of pulmonary artery stenosis and hypoplasia may be divided into congenital, acquired, and postsurgical. Pulmonary artery stenosis often complicates management of tetralogy of Fallot and pulmonary atresia with ventricular septal defect. As such, it may lead to significant maldistribution of pulmonary blood flow, right heart failure and right ventricular hypertension, as well as increased resistance to flow across the total pulmonary bed. The maintenance of low resistance to pulmonary blood flow is critical after Fontan-type operation. Branch pulmonary artery stenosis remains a difficult therapeutic problem. Surgical approaches are of limited success in addressing pulmonary artery stenoses, since the scarring and stenosis addressed by the procedure is replaced with scarring from the procedure. Stenoses distal to the hilum of the lung are difficult to reach surgically, and incomplete relief of more proximal lesions may contribute to operative morbidity and mortality (71,93). Balloon dilation of the affected vessel is clinically well tolerated and should be considered as the initial form of intervention. It carries a low morbidity risk and 1% to 2% mortality (18). Although angiographic improvement in vessel caliber (50% increase in vessel diameter or a 20% decrease in the systolic right ventricular to aortic pressure ratio) can be achieved in half the patients (58,68), restenosis is common (15% to 20%), and longterm clinical benefit is achieved in <35% of patients (59,115). There is some evidence that using balloons that can be inflated with high intra-balloon pressures may improve the success rate (40). Besides fatal or nonfatal pulmonary perforation, complications have included aneurysm formation and rarely unilateral pulmonary edema, which may be life threatening (4,40,59,115). Dilation of Systemic Venous Obstructions Venous stenoses can pose a significant problem as part of congenital, postoperative, or acquired cardiovascular disease. In the pediatric population, systemic venous obstructions are most often acquired after surgical procedures - for example, the atrial switch procedure for d-transposition of the great arteries (baffle obstruction occurring in up to 10-30% of patients), superior caval vein to pulmonary artery anastomosis, and Fontan operation (179). Baffle stenosis can result in venous congestion, pulmonary edema, reduced exercise tolerance, and heart failure. Balloon angioplasty of venous lesions has been successful acutely and carries little risk (107,179). The large caliber and significant elastic 10 recoil of venous channels, even in children, requires very large balloons for dilation and often double balloon techniques are employed for an effective angioplasty. Failure of primary angioplasty to provide lasting relief of stenosis is an indication for use of endovascular stent in the treatment of venous stenoses. Dilation of Miscellaneous Lesions Balloon dilation has been applied in the setting of fibromuscular dysplasia and Takayasu’s arteritis with partial success (88). Systemic-to-pulmonary artery shunts (both classical and modified Blalock-Taussig shunts) have been dilated successfully, with significant improvement in oxygen saturations (87,89,116). The application of endovascular stents may also be useful in these situations. Surgical backup support must be available when any type of shunt is dilated because of the danger of thrombosis or dislodgment of prosthetic intimal lining resulting in complete obstruction to flow. Endovascular stents A significant concern with vascular dilation procedures is resultant recurrence of the lesion, either acutely (distendable, but due to elastic recoil recurrent) or over longer periods (recurrent), despite an adequate initial dilation. Various intravascular stent devices have been developed to provide a framework to resist the elastic recoil in such vascular stenoses after failed or recurrent balloon angioplasty (112,114), providing vessel wall support. There are 2 major types of stents, balloonexpandable and self-expanding. In pediatric applications, the most commonly used stent implant is a balloon expandable stainless steel stent. This implant retains its size and shape after balloon expansion and can be further enlarged with increasing balloon diameters. Metal struts providing support rapidly become endothelialized (2 to 3 months) after implantation. Portions of metal, not apposed to the vessel wall remain uncovered, and vessel side branches off the stented lumen have been shown to remain patent (13). The largest pediatric experience is with stenting of congenital or postoperative branch pulmonary artery stenoses. These lesions are difficult if not impossible to access surgically, and the rate of restenosis after attempted surgery has been high. Success rate of balloon angioplasty of such lesions is only in the range of 50%, and restenosis occurs in up to 17%. With stent implantation, successful vessel enlargement is nearly 100% and the incidence of medium-term restenosis very low (59,170). In a multi-institutional study with 121 stent placements in 85 patients, there was immediate hemodynamic improvement (gradient reduction, vessel enlargement) and substantial increase in flow to the affected lung in all 11 patients. Midterm follow-up revealed restenosis in only 1 patient with successful redilation (113). Distal branch pulmonary artery stenosis is also amenable to treatment with multiple stent placements, and the stents can be overlapped in series to afford support of long segment stenoses. Such applications have been shown the most cost-effective means available in the treatment of branch pulmonary artery stenosis (170). While venous stenoses have been dilated with standard balloon dilation techniques, as with some pulmonary arteries, such stenoses can recoil to their original configuration as the balloon is withdrawn. Stent implantation has been successfully applied to these central venous stenoses in pediatric patients with excellent results and a low incidence of complications (112,179). If external compression is present, as in malignancy, endovascular stenting is preferable to angioplasty for longer lasting relief. In addition, stenting should be considered after recurrent stenosis following angioplasty, or as primary therapy in selected patients, who are of a size that would allow the stent to be dilated to a diameter close to that of an adult vessel. Stent repair of CoA may be useful in preventing restenosis and aneurysm formation seen after surgery or balloon angioplasty. Experience from both experimental and clinical studies suggest that aortic wall damage may be reduced by implanting the stent to a diameter slightly less than would have been chosen for simple balloon dilation (105,137,164). In the older child and adult, percutaneous endovascular stent implantation is becoming an attractive clinical alternative to dilation alone, since further intervention is not likely to be needed with the fully expanded device. However, further experience and follow-up studies are needed to elucidate the long-term outcomes of patients with an implanted stent in the aorta. Finally, transcatheter placement of stents has also been reported in the arterial duct, for palliation of complex congenital heart disease (42,146), in stenotic aortopulmonary collateral arteries, and for treatment of stenotic right ventricle-to-pulmonary artery conduits (113). Potential complications of stent placement include embolization or misplacement of stents, and rarely side branch narrowing or fracture of the stent struts (114). A large delivery sheath is required for implanting the majority of stent diameters. This may cause vascular complications, which is of major concern when implanting a stent (via the femoral artery) to treat CoA. Furthermore, the stent, especially when implanted into small vessels, may become obstructive from acute thrombosis or neointimal proliferation through the stent struts. The use of rigid stents in growing children has certain limitations. Growth of the child and the great vessel proximal and distal to the fixed diameter rigid stent will ultimately result in an acquired stenosis. Therefore, the stents should preferably be implanted at a size which will be sufficient for the patient as 12 an adult. However, re-expansion of a stent that has become incorporated into the vessel wall may be possible (49,104). New designs which allow an implanted stent to open (break apart) upon redilation to larger diameters are being evaluated (61). Opening of atrial communications Balloon atrial septostomy Balloon atrial septostomy was first described by Rashkind and Miller in 1966 (132) as a palliative procedure for transposition of the great arteries. Since then the technique has been successfully applied in a variety of clinical settings in which obstruction to flow at the atrial level may occur such as in total anomalous pulmonary venous connection, mitral valve and tricuspid valve atresias and pulmonary atresia with intact ventricular septum. The procedure relieves atrial obstruction to flow, enhances bidirectional mixing of the pulmonary and systemic venous blood, improves oxygen saturation, and leads to dramatic improvement in the infant’s hemodynamic and symptomatic status. The efficacy and safety of this procedure have been repeatedly demonstrated (133,157). Balloon atrial septostomy can be done from both the umbilical or femoral vein unless there is a congenital absence or acquired blockage of the inferior vena cava. Conventionally, the procedure is performed in the catheterization laboratory under fluoroscopic guidance (178) although, recently, several studies have demonstrated the feasibility and safety of bedside septostomy (10,27). The balloon catheter is advanced through the right atrium and foramen ovale into the left atrium. While continually observed on fluoroscopy or echocardiography, the balloon is dilated with diluted contrast and pulled rapidly across the atrial septum into the right atrium. The procedure is repeated until no resistance to withdrawal of the balloon is encountered. After the procedure, there is immediate equalization of pressures across the atrial septum. Complications include rhythm disturbances, perforation of the heart, embolization of the fragments of the balloon if ruptured, and damage of the atrioventricular valves, or systemic or pulmonary veins. Because septal thickness increases with age, balloon atrial septostomy is effective only in infants less than 1 to 2 months of age. Blade Septostomy In infants over 1 month of age, the atrial septum is usually too thick for a balloon septostomy to create an atrial septal defect. For these situations, a blade catheter that creates a tear in the atrial septum has been developed (119). The indications for blade septostomy are the same as for balloon septostomy. The 13 blade septostomy is always followed by balloon septostomy leading to equalization of pressures between the 2 atria. The procedure has been found safe and effective in patients of all age and size groups. Complications include air and clot embolism, perforation of the right atrium and ventricle and inability to retract the blade into the catheter (1). New balloon designs with blades have been used to initiate tears in the wall, and static balloon dilation, with single or multiple balloons have also been used in this situation. Closure of Extracardiac and Intracardiac Communications There are a variety of congenital and acquired anomalous vascular connections that require closure for relief of the hemodynamic burden, or to facilitate corrective surgical procedures. Percutaneous transcatheter occlusion of unwanted vascular communications was first described by Gianturco and coworkers more than 20 years ago (41). Today, the procedure is accomplished with implantation of a wide variety of occluding devices or materials to promote clot formation, with subsequent ingrowth of fibrous tissue, such as metal coils, detachable balloons, a gelatin-like sponge, polyvinyl alcohol particles, umbrellas, and detachable sacks (144). Gianturco coils (Cook, Bloomington, IN) are the most commonly used occlusion devices for children with congenital cardiac defects. They are small helical spring wires with fabric strands, available in multiple coil diameters, lengths, and wire thicknesses. Occlusion devices have been utilized to occlude aortopulmonary collateral vessels, small and moderate sized patent arterial ducts, surgically created Blalock-Taussig shunts, pulmonary and systemic arteriovenous fistulae, coronary arteriovenous fistulae, and anomalous venovenous connections (120,154). The technique of the embolization varies depending on the type of embolic material used, type of vascular connection to be occluded, and the specific pathophysiology. In general, such occlusion techniques are simple, the results are good, and the complication rates are low. The most common complications include malembolization into the distal pulmonary or systemic arteries. The embolized devices can usually be removed with retrieval devices or catheters. Complications less often encountered include unintentional occlusion of additional vessels, hemolysis, myocardial perforation, and vascular trauma (144). Closure of Aortopulmonary Collaterals Aortopulmonary collaterals arise directly from the aorta and supply segments of the lung, either directly (sole supply) or in parallel (dual supply) along with normally connected central pulmonary arteries. 14 These vessels are thin-walled and have segmental stenosis and areas of dilatation. They occur most frequently in children with tetralogy of Fallot or pulmonary atresia with VSD, and increase pulmonary blood flow and systemic ventricular volume load. Ligation of the collaterals is recommended at the time of the corrective surgery, but this can be technically difficult and time consuming, and at times impossible from a median sternotomy. If occlusion is attempted in the catheterization laboratory, the vessel must be test-occluded to assure adequate arterial saturation, and angiographic confirmation that the distal lung has central pulmonary artery supply (120). From several reported series (120,144), complete occlusion has been accomplished in 70% to 80% of the patients, and subtotal or partial occlusion in 10% to 25%, with a 2% to 11% failure rate, although late occlusion can occur in subtotally occluded vessels (120). Recanalization has been documented in some previously completely occluded vessels. Closure of Surgical Systemic to Pulmonary Shunts Surgical shunts can provide a reliable source of pulmonary blood flow, and are most often constructed of expanded polytetrafluoroethylene (Gore-Tex®, Phoenix, AZ, USA) between the subclavian and pulmonary arteries, or infrequently from the ascending aorta to the pulmonary artery (the so-called central shunt). Transcatheter shunt closure is preferable if shunt closure is the only indication for surgery, and particularly so for those left-sided shunts, known to be difficult to close surgically. Residual shunt flow detected in a shunt that has been surgically ligated is an additional indication for catheter intervention. For closure of surgical shunts, coils, balloons, and double disc umbrellas have been used (120,168). During these occlusion procedures, device migration can occur due to the high blood flow velocity through the shunt, and flow control is often required. In the reported series (19,120), complete closure rate has varied between 43% and 82%, although recent device improvements have resulted in occlusion rates near 100%. Closure of Arteriovenous Malformations Systemic and pulmonary arteriovenous malformations can lead to cyanosis and/or heart failure. They may be congenital or acquired following surgery such as a cavopulmonary anastomosis (superior caval vein to pulmonary artery shunt) or a modified Fontan procedure (171). Transcatheter embolization of these lesions has been well established as first line of therapy, particularly in the setting of multiple lesions. Frequently, several coils are required to occlude these lesions, with success rates varying between 80% and 90%. The complication rate is low, and recurrence rates in the range of 13% 15 (167,144). In hepatic and pulmonary arteriovenous malformations, feeding vessels can reopen or further abnormal vessels are recruited resulting in reappearance of the lesion. Coronary artery fistulas are rare, and usually isolated anomalies. They commonly drain into the right ventricle or right atrium. Most coronary fistulas should be closed to prevent infective endocarditis, congestive heart failure, or myocardial ischemia. They can be effectively and relatively safely closed with transcatheter coil occlusion techniques (121,139). Coil occlusion is most successful if a single arterial feeding vessel is present. Closure of Venovenous Connections In children treated with a cavopulmonary connection (Glenn shunt) or a Fontan procedure, abnormal connections from the systemic venous circulation, commonly from the neo-right atrium, azygos, or hemiazygos system and pericardial veins, draining to the pulmonary venous atrium can develop. Many of these communications may be present preoperatively albeit small as normal channels draining into the right atrium, enlarging with the increased atrial pressure after surgery. These communications provide a site for right to left shunting and decrease the volume of effective pulmonary blood flow (unoxygenated blood flow to the lungs). Many or most of these lesions can be closed by transcatheter embolization techniques (60). Surgical fenestrations after the Fontan procedure have also been successfully closed with double disc umbrella devices (16,138) or coils (158). The introduction of these methods has lead to significant improvements in the postoperative course of patients with complex congenital heart lesions (16,60,73). Closure of PDA The incidence of isolated patent arterial duct in full-term infants is about 1 in 2000 live births, accounting for approximately 10% of all types of congenital heart disease. Factors determining the clinical features in patients with a patent arterial duct include the size of the communication, the ratio of pulmonary and systemic vascular resistances, and the reserve of the ventricle to accommodate the extra volume load. Indications for transcatheter closure are the same as those for surgical closure, an audible continuous murmur with Doppler confirmation. Closure of the so-called silent ducts is controversial (2). The early devices utilized for closure are double disc umbrella devices (Rashkind PDA Occluder, CR BARD, Billerica, MA, USA) (58,79), the Botalloccluder, the buttoned device (Custom Medical Devices, Amarillo, Texas, USA) (130) and a coil filled bag (Gianturco-Grifka Vascular Occlusion Device, Cook Inc., Bloomington IN, USA) (50). The implantation techniques for these devices are 16 relatively complex, requiring large delivery catheters and each relatively expensive. Therefore, their use is generally reserved for large ducts only. With these devices, complete occlusion is frequent, only 3% to 14% of patients having persistent residual leaks in the follow-up after implantation (50,58,79,130). In small and moderate sized ducts, embolization using coils (Cook Inc., Bloomington, IN, USA) (20) some with detachable mechanisms (Duct-Occlud, PFM, Bonn, Germany; Target Therapeutics, Fremont, CA, USA) (169,173) has been shown to be effective, economical and safe (31). The procedure can be performed either from an anterograde or retrograde approach using small sheaths and catheters. The diameter of the coil should be at least twice the measured minimal ductal diameter. The use of multiple coils has been advocated to occlude larger ducts and to close residual ducts after previously attempted catheter closure with other devices or post ligation ducts. Successful implantation rate is in the range of 95% (150,151). Tiny residual shunts, occasionally noted immediately after coil implantation, often close spontaneously with complete occlusion rates between 90-100% (56,102,150). Embolization of the device during delivery has been the only major complication, with catheter retrieval of the embolized device possible in the vast majority of patients. Very rarely, hemolysis has been described in association with significant residual shunting (151). After the procedure, prophylaxis against subacute bacterial endocarditis is recommended for 6 months if occlusion is complete, and indefinitely if there is a persistent residual shunt. Closure of ASD and VSD Atrial septal defects that result in significant right ventricular volume overload should be closed if detected in children and young adults. With closure of the atrial defect the left to right shunt is eliminated, the total cardiac work, pulmonary blood flow, and volume loading of the right heart reduced, and potential associated arrhythmias lessened. In addition, a pathway for a paradoxical embolus is eliminated. The first transcatheter attempt to close an atrial defect occurred in the 1970s, when King and coworkers reported the use of a double-umbrella device in humans (70). Since that time, modifications of techniques and devices have improved the applicability and results of percutaneous closure in this setting. Currently, there are 5 types of devices available for transcatheter closure, and they include the Amplatzer (Company?, Minneapolis, MN) (91), the Angel Wings (Microvena Corp, Vadnais, MN, USA) (25), the ASDOS (Osypka Corporation, Rheinfelden, Germany) (52), the buttoned device (Custom Medical Devices, Amarillo, Texas, USA) (44,155), and the CardioSeal implant (Nitinol Medical Technologies, Inc, Boston, MA, USA) (14,65). While several devices have self-centering mechanisms (25,91,155) all are designed to be applied to a remnant of existing atrial septum. Therefore 17 they must be significantly larger than the defect itself, and they must be precisely positioned if the remaining septal rim is not large. Transesophageal echocardiography has been found useful during device placement to visualize the atrial defect (14). These devices are applicable to patients with a secundum type of atrial defect. None of the implants are applicable to patients with very large defects or to patients with sinus venosus or ostium primum type defects. The technique of device implantation varies according to the device chosen. In general, however all include angiographic imaging and balloon sizing of the defect. The device is then loaded into the catheter and delivered through the defect, with positioning, release, and fixation of the device guided by transesophageal echocardiography and fluoroscopy. Immediately after the device implantation, small residual leaks are frequently seen by color Doppler in the majority of patients. Spontaneous resolution of residual shunting is common, and after 1 year, hemodynamically significant leaks can be found in only 5% to 10% of patients (14,65,80). Complications include embolization of the device or part of it, if inappropriately placed on the atrial septum. Additionally, the rigid frames of these devices can fracture with time. The device may also disturb other intracardiac structures such as the atrioventricular valves causing regurgitation, obstruction of the systemic or pulmonary veins, or protrusion into the great arteries causing perforation of the aorta or pulmonary artery. These problems are fortunately however very rare occurrences. Transcatheter closure techniques have been applied to both congenital and acquired forms of ventricular septal defects in attempt to eliminate the need for, or reduce the risk and complexity of surgical repair (17). In small infants with muscular VSDs and other complex defects, intraoperative device closure may be beneficial (33). The Clamshell device (CR BARD, Billerica, MA - no longer available) (83), the Rashkind double umbrella device (CR BARD, Billerica, MA) (140) and buttoned devices (Custom Medical Devices, Amarillo, Texas, USA) (156) have been used to close muscular and perimembranous ventricular defects with variable success. Further experience, better designed implants, and follow-up studies are needed to define the safety and efficacy of this procedure. Summary Over the past decade, transcatheter interventions have become increasingly important in the treatment of patients with congenital heart lesions. These procedures may be broadly grouped as dilations (septostomy, valvuloplasty, angioplasty, and endovascular stenting) or as closures (vascular embolization and device closure of defects). Balloon valvuloplasty has become the treatment of choice 18 for simple valvar pulmonic stenosis in all age groups and, although not curative, appears at least comparable to surgery for congenital aortic stenosis in newborns through young adults. Balloon angioplasty is successfully applied to a wide range of aortic, pulmonary artery and venous stenoses. Stents are useful in dilating lesions whose intrinsic elasticity results in vessel recoil after balloon dilation alone. Catheter delivered coils are used to embolize a wide range of arterial, venous, and prosthetic vascular connections. Although some devices remain at present investigational, they have been successfully used for closure of a large number of arterial ducts as well as atrial and ventricular septal defects. In the therapy of the patient with complex congenital heart disease, best results may be achieved by combining cardiac surgery with interventional catheterization. The co-operation between interventional cardiologist and cardiac surgeon was recently highlighted in a report of an algorithm to manage the patient with tetralogy of Fallot or pulmonary atresia with diminutive pulmonary arteries, involving balloon dilation, coil embolization of collaterals, and intraoperative stent placement (75). In this setting, well planned catheterization procedures can play an important role in reducing the overall number of procedures a patient may require over a lifetime, with improved outcomes. 19 References 1. Ali Khan MA, Bricker JT, Mullins CE, et al: Blade atrial septostomy: Experience with the first 50 procedures. Cathet Cardiovasc Diagn 23:257, 1991 2. Allen HD, Beekman RH III, Garson A Jr, et al: Pediatric therapeutic cardiac catheterization. A statement for healthcare professionals from the Council on Cardiovascular Disease in the Young, American Heart Association. Circulation 97:609, 1998 3. Anjos R, Quereshi SA, Rosenthal E. et al: Determinants of hemodynamic results of balloon dilatation of aortic recoarctation. Am J Cardiol 69:665, 1992 4. Arnold LW, Keane JF, Kan JS, et al: Transient unilateral pulmonary edema after successful balloon dilation of peripheral pulmonary artery stenosis. Am J Cardiol 62:327, 1988 5. Balis JU, Chan AS, Conen PE: Morphogenesis of human aortic coarctation. Exp Mol Pathol 6:25, 1967 6. Beekman RH III, Rocchini Behrendt DM, Rosenthal A: Reoperation for coarctation of the aorta. Am J Cardiol 48:1108, 1981 7. Beekman RH III, Rocchini AP, Behrendt DM, et al: Long-term outcome after repair of coarctation in infancy: Subclavian angioplasty does not reduce the need for reoperation. J Am Coll Cardiol 8:1406, 1986 8. Beekman RH III, Rocchini AP, Dick M, et al: Percutaneous balloon angioplasty for native coarctation of the aorta. J Am Coll Cardiol 10:1078, 1987 9. Beekman RH III, Rocchini AP, Andes A: Balloon valvuloplasty for critical aortic stenosis in the newborn: influence of new catheter technology. J Am Coll Cardiol 17:1172, 1991 10. Beitzke A, Stein JI, Suppan C: Balloon atrial septostomy under two-dimensional echocardiographic control. Int J Cardiol 30:33, 1991 11. Ben-Shachar G, Cohen MH, Sivakof MC et al: Development of infundibular obstruction after percutaneous pulmonary balloon valvuloplasty. J Am Coll Cardiol 5:754, 1985 12. Benson LN, Smallhorn JS, Freedom RM et al: Pulmonary valve morphology after balloon dilatation of pulmonary valve stenosis. Cathet Cardiovasc Diagn 11:161, 1985 13. Benson LN, Hamilton R, Dasmahapatra H, et al: Percutaneous implantation of a balloonexpandable endoprosthesis for pulmonary artery stenosis: An experimental study. J Am Coll Cardiol 18:1303, 1991 14. Boutin C, Musewe NN, Smallhorn JF, et al: Echocardiographic follow-up of atrial septal defect after catheter closure by double-umbrella device. Circulation 88:621, 1993 20 15. Brandt B, Marvin WJ, Rose EF, et al: Surgical treatment of coarctation of the aorta after balloon angioplasty. J Thorac Cardiovasc Surg 94:715, 1987 16. Bridges ND, Lock JE, Castaneda AR: Baffle fenestration with subsequent transcatheter closure: Modification of the Fontan operation for patients at increased risk. Circulation 82:1681, 1990 17. Bridges ND, Perry SB, Keane JF, et al: Perioperative transcatheter closure of congenital muscular ventricular septal defects. N Engl J Med 321:1312, 1991 18. Burrows PE, Benson LN, Williams WG, et al: Iliofemoral arterial complications of balloon angioplasty for systemic obstructions in infants and children. Circulation 82:1697, 1990 19. Burrows PE, Edwards TC, Benson LN: Transcatheter occlusion of Blalock-Taussig shunts: technical options. J Vasc Intervent Radiol 4:673, 93 20. Cambier PA, Kirby WC, Wortham DC, et al: Percutaneous closure of small (<2.5mm) patent ductus arteriosus using coil embolization. Am J Cardiol 69:815, 1993 21. Caspi J, Coles JG, Benson LN, et al: Management of neonatal critical pulmonic stenosis in the balloon valvotomy era. Ann of Thorac Surg 49:273, 1990 22. Colli AM, Perry SB, Lock JE, et al: Balloon dilation of critical valvar pulmonary stenosis in the first month of life. Cath Cardiovasc Diagn 34:23, 1995 23. Collins-Nakai R, Rosenthal A, Castaneda A, et al: Congenital mitral stenosis: a review of 20 years’ experience. Circulation 56:1039, 1977 24. Cooper SG, Sullivan ID, Wren C: Treatment of recoarctation: Balloon dilation angioplasty. J Am Coll Cardiol 14:413, 1989 25. Das GS, Voss G, Jarvis G, et al: Experimental atrial septal defect closure with a new, transcatheter, self-centering device. Circulation 88:1754, 1993 26. DiSessa TG, Alpert BS, Chase NA et al: Balloon valvuloplasty in children with dysplastic pulmonary valves. Am J Cardiol 60:405, 1987 27. D’Orsogna L, Lam J, Sandor GG, et al: Assessment of bedside umbilical vein balloon septostomy using two-dimensional echocardiographic guidance in transposition of great arteries. Int J Cardiol 25:271, 1989 28. Edwards BS, Lucas RV, Lock JE, et al: Morphologic changes in the pulmonary arteries after percutaneous balloon angioplasty for pulmonary arterial stenosis. Circulation 71:195, 1985 29. Egito ES, Moore P, O'Sullivan J, et al: Transvascular balloon dilation for neonatal critical aortic stenosis: Early and midterm results. J Am Coll Cardiol 29:442, 1997 21 30. Fedderly RT, Lloyd TR, Mendelsohn AM, et al: Determinants of successful balloon valvotomy in infants with critical pulmonary stenosis or membranous pulmonary atresia with intact ventricular septum. J Am Coll Cardiol 25:460, 1995 31. Fedderly RT, Beekman RH III, Mosca RS, et al: Comparison of hospital charges for closure of patent ductus arteriosus by surgery and by transcatheter coil occlusion. Am J Cardiol 77:776, 1996 32. Feldman T, Chiu YC, Carroll JD: Catheter balloon dilatation for discrete subaortic stenosis in the adult. Am J Cardiol 60:403, 1987 33. Fishberger SB, Bridges ND, Keane JF, et al: Intraoperative device closure of ventricular septal defects. Circulation 88:II-205-II-209, 1993 34. Fisher DR, Ettedgui JA, Park SC, et al: Carotid artery approach for balloon dilation of aortic valve stenosis in the neonate: a preliminary report. J Am Coll Cardiol 15:1633, 1990 35. Fletcher SE, Nihill MR, Grifka RG, et al: Balloon angioplasty of native coarctation of the aorta: Midterm follow-up and prognostic factors. J Am Coll Cardiol 25:730, 1995 36. Fontes VF, Esteves CA, Sousa JEMR et al: Regression of infundibular hypertrophy after pulmonary valvuloplasty for pulmonic stenosis. Am J Cardiol 62: 977-979, 1988 37. Galal O, Rao PS, Al-Fadley F, et al: Follow-up results of balloon aortic valvuloplasty in children with special reference to causes of late aortic insufficiency. Am Heart J 133:418, 1997 38. Gatzoulis MA, Rigby ML, Shinebourne EA, et al: Contemporary results of balloon valvuloplasty and surgical valvotomy for congenital aortic stenosis. Arch Dis Child 73:66, 1995 39. Gaynor JW, Bull C, Sullivan ID, et al: Late outcome of survivors of intervention for neonatal aortic valve stenosis. Ann Thorac Surg 60:122, 1995 40. Gentles TL, Lock JE, Perry SB: High pressure balloon angioplasty for branch pulmonary artery stenosis: early experience. J Am Coll Cardiol 22:867, 1993 41. Gianturco C, Anderson JH, Wallace S: Mechanical devices for arterial occlusion. Am J Roentgenol Ther Nucl Med 124:428, 1975 42. Gibbs JL, Rothman MT, Rees MR, et al: Stenting of the arterial duct: A new approach to palliation for pulmonary atresia. Br Heart J 67:240, 1992 43. Gibbs JL, Blackburn ME, Uzun O, et al: Laser valvotomy with balloon valvoplasty for pulmonary atresia with intact ventricular septum: Five years' experience. Heart 77:225, 1997 44. Gildein HP, Dabritz S, Geibel A, et al: Transcatheter closure of atrial septal defects by the "buttoned" device: Complications and need for surgical revision. Pediatr Cardiol 18:328, 1997 45. Gildein HP, Kleinert S, Goh TH, et al: Treatment of critical pulmonary valve stenosis by balloon dilatation in the neonate. Am Heart J 131:1007, 1996 22 46. Giusti S, Borghi A, Redaelli S, et al: The carotid arterial approach for balloon dilation of critical aortic stenosis in neonates - immediate results and follow-up. Cardiol Young 5:155, 1995 47. Gournay V, Piechaud JF, Delogu A, et al: Balloon valvotomy for critical stenosis or atresia of pulmonary valve in newborns. J Am Coll Cardiol 26:1725, 1995 48. Grifka RG, O’Laughlin MP, Nihill MR, et al: Double-transseptal, double-balloon valvuloplasty for congenital mitral stenosis. Circulation 85:123, 1992 49. Grifka RG, Vick GW, O'Laughlin MP, et al: Balloon-expandable intravascular stents: Aortic implantation and late further dilation in growing mini-pigs. Am Heart J 126:979, 1993 50. Grifka RG, Vincent JE, Nihill MR, et al: Transcatheter patent ductus arteriosus closure in an infant using the Gianturco-Grifka vascular occlusion device. Am J Cardiol 78:721, 1996 51. Gundry SR, Razzouk AJ, Boskind JF, et al: Fate of the pericardial monocusp pulmonary valve for right ventricular outflow tract reconstruction. Early function, late failure without obstruction.. Thorac Cardiovasc Surg 107:908, 1994 52. Hausdorf G, Schneider M, Franzbach B, et al: Transcatheter closure of secundum atrial septal defects with the atrial septal defect occlusion system (ASDOS): Initial experience in children. Heart 75:83, 1996 53. Hawkins JA, Minich LL, Shaddy RE, et al: Aortic valve repair and replacement after balloon aortic valvuloplasty in children. Ann Thorac Surg 61:1355, 1996 54. Hellenbrand WE, Allen HD, Golinko RJ, et al: Balloon angioplasty for aortic recoarctation: results of valvuloplasty and angioplasty of congenital anomalies registry. Am J Cardiol 65:793, 1990 55. Hijazi ZM, Fahey JT, Kleinman CS, et al: Balloon angioplasty for recurrent coarctation of aorta: Immediate and long term results. Circulation 84:1150, 1991 56. Hijazi ZM, Geggel RL: Results of anterograde transcatheter closure of patent ductus arteriosus using single or multiple Gianturco coils. Am J Cardiol 74:925, 1994 57. Ho, SY, Somerville J, Yip WCL, et al: Transluminal balloon dilation of resected coarcted segments of thoracic aorta: Histological study and clinical implications. Int J Cardiol 19:99, 1988 58. Hosking MCK, Benson LN, Musewe N, et al: Transcatheter occlusion of the persistently patent ductus arteriosus: Forty-month follow-up and prevalence of residual shunting. Circulation 84:2313, 1991 59. Hosking MCK, Thomaidis C, Hamilton R, et al: Clinical impact of balloon angioplasty for branch pulmonary arterial stenosis. Am J Cardiol 69:1467, 1992 23 60. Hsu HS, Nykanen DG, Williams WG, et al: Right to left interatrial communications after the modified Fontan procedure: Identification and management with transcatheter occlusion. Br Heart J 74:549, 1995 61. Ing FF, Fagan TE, Kearney DL, et al: A new “open-ring” stent (abstr). Circulation 94:I-57, 1996 62. Isner JM, Donaldson RF, Fulton D, et al: Cystic medial necrosis in coarctation of the aorta: A potential factor contributing to adverse consequences observed after percutaneous balloon angioplasty of coarctation sites. Circulation 75:689, 1987. 63. Joseph PK, Bhat A, Francis B, et al: Percutaneous transvenous mitral commissurotomy using an Inoue balloon in children with rheumatic mitral stenosis. Int J Cardiol 62:19, 1997 64. Justo RN, McCrindle BW, Benson LN, et al: Aortic valve regurgitation after surgical versus percutaneous balloon valvotomy for congenital aortic valve stenosis. Am J Cardiol 77:1332, 1996 65. Justo RN, Nykanen DG, Boutin C, et al: Clinical impact of transcatheter closure of secundum atrial septal defects with the double umbrella device. Am J Cardiol 77:889, 1996 66. Justo RN, Nykanen D, Williams WG, et al: Transcatheter perforation of the right ventricular outflow tract as initial therapy for pulmonary valve atresia and intact ventricular septum in the newborn. Cath Cardiovasc Diagn 40:408, 97 67. Kan JS, White RI, Mitchell SE, et al: Percutaneous balloon valvuloplasty: A new method for treating congenital pulmonary valve stenosis. N Engl J Med 307:540, 1982 68. Kan JS, Marvin WJ, Bass JL, et al: Balloon angioplasty-branch pulmonary artery stenosis: Results from the Valvuloplasty and Angioplasty of Congenital Anomalies Registry. Am J Cardiol 65:798, 1990 69. Kasten-Sportes CH, Piechaud JF, Sidi D, et al: Percutaneous balloon valvuloplasty in neonates with critical aortic stenosis. J Am Coll Cardiol 13:1101, 1989 70. King TD, Thompson SL, Steiner C, et al: Secundum atrial septal defect: Nonoperative closure during cardiac catheterization. JAMA 235:2506, 1976 71. Kirklin JW, Blackstone EH, Kirklin JK, et al: Surgical results and protocols in the spectrum of tetralogy of Fallot. Ann Surg 198:251, 1983 72. Kopf GS, Geha AS, Hellenbrand WE, et al: Fate of left-sided cardiac bioprosthesis valves in children. Arch Surg 121:488, 1986 73. Kopf GS, Kleinman CS, Hijazi ZM, et al: Fenestrated Fontan operation with delayed transcatheter closure of atrial septal defect. Improved results in high-risk patients. J Thorac Cardiovasc Surg 103:1039, 1992 74. Kovalchin JP, Forbes TJ, Nihill MR, et al: Echocardiographic determinants of clinical course in infants with critical and severe pulmonary valve stenosis. J Am Coll Cardiol 29:1095, 1997 24 75. Kreutzer J, Perry SB, Jonas RA, et al: Tetralogy of Fallot with diminutive pulmonary arteries: Preoperative pulmonary valve dilation and transcatheter rehabilitation of pulmonary arteries. J Am Coll Cardiol 27:1741, 1996 76. Kuhn MA, Latson LA, Cheatham JP, et al: Management of pediatric patients with isolated valvar aortic stenosis by balloon aortic valvuloplasty. Cath Cardiovasc Diagn 39:55, 1996 77. Lababidi Z, Wu JR: Percutaneous balloon pulmonary valvuloplasty.Am J Cardiol 52:560, 1983 78. Ladusans EJ, Qureshi SA, Parsons JM, et al: Balloon dilatation of critical stenosis of the pulmonary valve in neonates. Br Heart J 63:362, 1990 79. Latson LA, Hofschire PJ, Kugler JD, et al: Transcatheter closure of patent ductus arteriosus in pediatric patients. J Pediatr 115:549, 1989 80. Latson LA: Per-catheter ASD closure. Pediatr Cardiol 19:86, 1998 81. Lock JE, Keane JF, Fellows KE: Interventional cardiac catheterization. In Macartney FJ (ed): Congenital Heart Diseases, Lancaster, M.T.P. Press Ltd., 1986, p 163 82. Lock JE, Keane JF, Fellows KE: The use of catheter intervention procedures for congenital heart disease. J Am Coll Cardiol 7:1420, 1986 83. Lock JE, Block PC, McKay RG, et al: Transcatheter closure of ventricular septal defects. Circulation 78:361, 1988 84. Maeno Y, Akagi T, Hashino K, et al: Carotid artery approach to balloon aortic valvuloplasty in infants critical aortic valve stenosis. Pediatr Cardiol 18:288, 1997 85. Magee AG, Nykanen D, McCrindle BW, et al: Balloon dilation of severe aortic stenosis in the neonate: Comparison of anterograde and retrograde catheter approaches. J Am Coll Cardiol 30:1061, 1997 86. Marantz PM, Huhta JC, Mullins CE et al: Results of balloon valvuloplasty in typical and dysplastic pulmonary valve stenosis: Doppler echocardiographic follow-up. J Am Coll Cardiol 12:476, 1988 87. Marks LA, Mehta AV, Marangi D: Percutaneous transluminal balloon angioplasty of stenotic standard Blalock-Taussig shunts: Effect on choice of initial palliation in cyanotic congenital heart disease. J Am Coll Cardiol 18:546, 1991 88. Martin EC, Diamond NG, Casarella WJ: Percutaneous transluminal angioplasty in nonatherosclerotic disease. Radiology 135:27, 1980 89. Marx GR, Allen HD, Ovitt TW, et al: Balloon dilation angioplasty of Blalock-Taussig shunts. Am J Cardiol 62:824, 1988 25 90. Masura J, Burch M, Deanfield JE, et al: Five-year follow-up after balloon pulmonary valvuloplasty. J Am Coll Cardiol 21:132, 1993 91. Masura J, Gavora P, Formanek A, et al: Transcatheter closure of secundum atrial septal defects using the new self-centering amplatzer septal occluder: Initial human experience. Cath Cardiovasc Diagn 42:388, 1997 92. Matsuoka S, Ushiroguchi Y, Kubo M, et al: Balloon pulmonary valvuloplasty for infants with severe tetralogy of Fallot. Japn Heart J 34:643, 1993 93. Mayer JE Jr, Helgason H, Jonas RA, et al: Extending the limits for modified Fontan procedures. J Thorac Cardiovasc Surg 92:1021, 1986 94. McCrindle BW, Kan JS: Long-term results after balloon pulmonary valvuloplasty. Circulation 83:1915, 1991 95. McCrindle BW, VACA Registry Investigators: Independent Predictors of long-term results after balloon pulmonary valvuloplasty. Circulation 89:1751, 1994 96. McCrindle BW: Independent predictors of immediate results of percutaneous balloon aortic valvotomy in children. Valvuloplasty and Angioplasty of Congenital Anomalies (VACA) Registry Investigators. Am J Cardiol 77:286, 1996 97. McCrindle BW, Jones TK, Morrow WR, et al: Acute results of balloon angioplasty of native coarctation versus recurrent aortic obstruction are equivalent. J Am Coll Cardiol 28:1810, 1996 98. McKay RG, Lock JE, Safian RD et al: Balloon dilation of mitral stenosis in adult patients: Postmortem and percutaneous mitral valvuloplasty studies. J Am Coll Cardiol 9:723, 1987 99. Mendelsohn AM, Lloyd TR, Crowley DC, et al: Late follow-up of balloon angioplasty in children with native coarctation of the aorta. Am J Cardiol 74:696, 1994 100. Miller DC, Stinson EB, Oyer PE, et al: The durability of porcine xenograft valves and conduits in children. Circulation 66(suppl):I-172, 1982 101. Moore P, Adatia I, Spevak P, et al: Severe congenital mitral stenosis in infants. Circulation 89:2099, 1994 102. Moore JW, George L, Kirkpatrick SE, et al: Percutaneous closure of the small patent ductus arteriosus using occluding spring coils. J Am Coll Cardiol 23:759, 1994 103. Moore P, Egito E, Mowrey H, et al: Midterm results of balloon dilation of congenital aortic stenosis: Predictors of success. J Am Coll Cardiol 27:1257, 1996 104. Morrow WR, Palmaz JC, Tio FO, et al: Re-expansion of balloon-expandable stents after growth. J Am Coll Cardiol 22: 2007, 1993 26 105. Morrow WR, Smith VC, Ehler WJ, et al: Balloon Angioplasty with stent implantation in experimental coarctation of the aorta. Circulation 89: 2677, 1994 106. Mosca RS, Iannettoni MD, Schwartz SM et al: Critical aortic stenosis in the neonate. A comparison of balloon valvuloplasty and transventricular dilation. J Thorac Cardiovasc Surg 109:147, 1995 107. Mullins CE, Latson LA, Neches WH, et al: Balloon dilatation of miscellaneous lesions: Results of Valvuloplasty and Angioplasty of Congenital Anomalies Registry. Am J Cardiol 65:802, 1990 108. Musewe NM, Robertson MA, Benson LN et al: The dysplastic pulmonary valve: Echocardiographic features and results of balloon valvuloplasty. Br Heart J 57:364, 1987 109. National Heart, Lung, and Blood Institute Balloon Valvuloplasty Registry: Complications and mortality of percutaneous balloon mitral commissurotomy. Circulation 85:2014, 1992 110. O’Connor BK, Beekman RH III, Rocchini AP et al: Intermediate-term effectiveness of balloon valvuloplasty for congenital aortic stenosis. A prospective follow-up study. Circulation 84:732, 1991 111. O'Connor BK, Beekman RH III, Lindauer A, et al: Intermediate-term outcome after pulmonary balloon valvuloplasty: Comparison with a matched surgical control group. J Am Coll Cardiol 20:169, 1992 112. O'Laughlin MP, Perry SB, Lock JE, et al: Use of endovascular stents in congenital heart disease. Circulation 83:1923, 1991 113. O'Laughlin MP, Slack MC, Grifka RG, et al: Implantation and intermediate-term follow-up of stents in congenital heart disease. Circulation 88:605, 1993 114. O’Laughlin MP: Balloon-expandable stenting in pediatric cardiology. J Intervent Cardiol 8:463, 1995 115. O’Laughlin MP: Catheterization treatment of stenosis and hypoplasia of pulmonary arteries. Pediatr Cardiol 19:48, 1998 116. Ormiston JA, Neutze JM, Calder AL, et al: Percutaneous balloon angioplasty for early postoperative modified Blalock-Taussig shunt failure. Cathet Cardiovasc Diagn 29:31, 1993 117. Ovaert C, Benson LN, Nykanen D, et al: Transcatheter treatment of coarctation of the aorta: A review. Pediatr Cardiol 19:27, 1998 118. Pagani FD, Cheatham JP, Beekman RH III, et al: The management of tetralogy of Fallot with pulmonary atresia and diminutive pulmonary arteries. J Thorac Cardiovasc Surg 110:1521, 1995 119. Park SC, Neches WH, Mullins CE, et al: Blade septostomy: collaborative study. Circulation 66:258, 1982 27 120. Perry SB, Radtke W, Fellows KE, et al: Coil embolization to occlude aortopulmonary collateral vessels and shunts in patients with congenital heart disease. J Am Coll Cardiol 13:100, 1989 121. Perry SB, Rome J, Keane JF, et al: Transcatheter closure of coronary artery fistulas. J Am Coll Cardiol 20:205, 1992 122. Qureshi SA, Reidy JF, Bin Alwi M, et al: Use of interlocking detachable coils in embolization of coronary arteriovenous fistulas. Am J Cardiol 78:110, 1996 123. Radtke W, Keane JF, Fellows KE et al: Percutaneous balloon valvotomy of congenital pulmonary stenosis using oversized balloons. J Am Coll Cardiol 8:909, 1986 124. Rao PS: Further observations on the role of balloon size on the short-term and intermediate term results of balloon pulmonary valvuloplasty. Br Heart J 60:507, 1988 125. Rao PS, Brais M: Balloon pulmonary valvuloplasty for congenital cyanotic heart defects. Am Heart J 115:1105, 1988 126. Rao PS, Thapar MK, Kutayli F: Causes of restenosis following balloon valvuloplasty for valvar pulmonary stenosis. Am J Cardiol 62:979, 1988 127. Rao PS: Indications for balloon pulmonary valvuloplasty. Am Heart J 116:1661, 1989 128. Rao PS, Wilson AD, Chopra PS: Immediate and follow-up results of balloon angioplasty of postoperative recoarctation in infants and children. Am Heart J 120:1315, 1990 129. Rao PS, Chopra PS: Role of balloon angioplasty in the treatment of aortic coarctation. Ann Thorac Surg 52:621, 1991 130. Rao PS, Ende DJ, Wilson AD, et al: Follow-up results of transcatheter occlusion of atrial septal defects with buttoned device. Can J Cardiol 11:695, 1995 131. Rao PS: Interventional pediatric cardiology: State of the art and future directions. Pediatr Cardiol 19:107, 1998 132. Rashkind WJ, Miller WW: Creation of an atrial septal defect without thoracotomy. JAMA 196:991, 1966 133. Rashkind WJ, Miller WW: Transposition of the great arteries: Results of palliation by balloon atrioseptostomy in thirty-one infants. Circulation 38:453, 1968 134. Rashkind WJ: A glance forward: Closure of cardiac defects without surgery. In Graham G, Rossi E (eds): Heart Disease in Infants and Children. London, Edward Arnold Ltd., 1980, p 249 135. Rashkind WJ: Transcatheter treatment of congenital heart disease. Circulation 67:711, 1983 136. Rashkind WJ, Gibson J Jr: Interventional cardiac catheterization in congenital heart disease. Int J Cardiol 7:1, 1985 28 137. Redington AN, Hayes AM, Ho SY: Transcatheter stent implantation to treat aortic coarctation in infancy. Br Heart J 69:80, 1993 138. Redington AN, Rigby ML. Transcatheter closure of interatrial communications with a modified umbrella device. Br Heart J 72:372, 1994 139. Reidy JF, Anjos RT, Qureshi SA, et al: Transcatheter embolization in the treatment of coronary artery fistulas. J Am Coll Cardiol 18:187, 1991 140. Rigby ML, Redington AN: Primary transcatheter umbrella closure of perimembranous ventricular septal defect. Br Heart J 72:368, 1994 141. Ring JC, Bass JL, Marvin W, et al: Management of congenital stenosis of a branch pulmonary artery with balloon dilation angioplasty: Report of 52 procedures. J Thorac Cardiovasc Surg 90:35, 1985 142. Rocchini AP, Beekman RH III, Ben Shachar G, et al: Balloon aortic valvuloplasty: Results of the Valvuloplasty and Angioplasty of Congenital Anomalies Registry. Am J Cardiol 65:784, 1990 143. Rome JJ: Balloon pulmonary valvuloplasty. Pediatr Cardiol 19: 18, 1998 144. Rothman A: Pediatric cardiovascular embolization therapy. Pediatr Cardiol 19:74, 1998 145. Rubio-Alvarez V, Limon-Larson R: Treatment of pulmonary valvular stenosis and tricuspid stenosis with a modified cardiac catheter. Program Abst II Second World Congress on Cardiology, Washington D.C., 1954, p 205 146. Ruiz CE, Gamra H, Zhang HP, et al: Stenting of the ductus arteriosus as a bridge to cardiac transplantation in infants with the hypoplastic left heart syndrome. N Eng J Med 328:1605, 1993 147. Saiki K, Kato H, Suzuki K, et al: Balloon valvuloplasty for congenital aortic valve stenosis in an infant and children. Acta Paediatr Japonica 34:433, 1992 148. Saxena A, Fong LV, Ogilvie BC, et al: Use of balloon dilatation to treat supravalvar pulmonary stenosis developing after anatomical correction for complete transposition. Br Heart J 64:151, 1990 149. Schoen FJ, Fernandez J, Gonzalez-Lavin L, et al: Causes of failure and pathologic findings in surgically removed Ionescu-Shiley standard bovine pericardial heart valve bioprothesis: emphasis on progressive structural deterioration. Circulation 76:618, 1987 150. Shim D, Fedderly RT, Beekman RH III, et al: Follow-up of coil occlusion of patent ductus arteriosus. J Am Coll Cardiol 28:207, 1996 151. Shim D, Beekman RH III: Transcatheter management of patent ductus arteriosus. Pediatr Cardiol 19:67, 1998 29 152. Sholler GF, Keane JF, Perry SB et al: Balloon dilation of congenital aortic valve stenosis. Results and influence of technical and morphological features on outcome. Circulation 78:351, 1988 153. Shrivastava S, Dev V, Vasan RS, et al: Percutaneous balloon mitral valvuloplasty in juvenile rheumatic mitral stenosis. Am J Cardiol 67:892, 1991 154. Siblini G, Rao PS: Coil embolization in the management of cardiac problems in children. J Invasive Cardiol 8:332, 1996 155. Sideris EB, Leung M,Yoon JH, et al: Occlusion of large atrial septal defects with a centering buttoned device: Early clinical experience. Am Heart J 131:356, 1996 156. Sideris EB, Walsh KP, Haddad GL, et al: The challenge of transcatheter membranous ventricular septal defect occlusion: Medium-term follow-up results with the buttoned device (abstr). Circulation 94:I-57, 1996 157. Singh SP, Astley R, Burrows FGO: Balloon septostomy for transposition of great arteries. Br Heart J 31:722, 1969 158. Sommer RJ, Recto M, Golinko RJ, et al: Transcatheter coil occlusion of surgical fenestration after Fontan operation. Circulation 94:249, 1996 159. Spevak PJ, Bass JL, Ben-Shachar G, et al: Balloon angioplasty for congenital mitral stenosis. Am J Cardiol 66:472, 1990 160. Spielenberg SR, Hutter PA, van de Wal HJ, et al: Late re-interventions following arterial switch operations in transposition of the great arteries. Incidence and surgical treatment of postoperative pulmonary stenosis. Eur J Cardiothoracic Surg 9:7, 1995 161. Sreeram N, Saleem M, Jackson M et al: Results of balloon pulmonary valvuloplasty as a palliation procedure in tetralogy of Fallot. J Am Coll Cardiol 18:156, 1991 162. Stanger P, Cassidy SC, Girod DA, et al: Balloon pulmonary valvuloplasty: Results of the Valvuloplasty and Angioplasty of Congenital Anomalies Registry. Am J Cardiol 65:775, 1990 163. Stumper O, Piechaud JF, Bonhoeffer P, et al: Pulmonary balloon valvuloplasty in the palliation of complex cyanotic congenital heart disease. Heart 76:363, 1996 164. Suarez de Lezo J, Pan M, Romero M, et al: Balloon-expandable stent repair of severe coarctation of aorta. Am Heart J 129:1002, 1995 165. Sweeney MS, Walker WE, Duncan JM: Reoperation for aortic coarctation: Techniques, results and indications for various approaches. Ann Thorac Surg 40:46, 1985 166. Tabatabaei H, Boutin C, Nykanen DG, et al: Morphologic and hemodynamic consequences after percutaneous balloon valvotomy for neonatal pulmonary stenosis: medium-term follow-up J Am Coll Cardiol 27:473, 1996 30 167. Terry PB, White RI Jr, Barth KH, et al: Pulmonary arteriovenous malformation: Physiologic observations and results of therapeutic balloon embolization. N Eng J Med 308:1197, 83 168. Tometzki AJ, Houston AB, Redington AN, et al: Closure of Blalock-Taussig shunts using a new detachable coil device. Br Heart J 73:383, 1995 169. Tometzki A, Chan K, De Giovanni J, et al: Total UK multicenter experience with a novel arterial occlusion device (Duct Occlud pfm). Heart 76:520, 1996 170. Trant CA Jr, O’Laughlin MP, Ungerleider RM, et al: Cost-effectiveness analysis of stents, balloon angioplasty, and surgery for the treatment of branch pulmonary artery stenosis. Pediatr Cardiol 18:339, 1997 171. Triedman JK, Bridges ND, Mayer JE, et al: Prevalence and risk factors for aortopulmonary collateral vessels after fontan and bidirectional Glenn procedures. J Am Coll Cardiol 22:207, 1993 172. Tynan M, Finley JP, Fontes V, et al: Balloon angioplasty for the treatment of native coarctation: Results of Valvuloplasty and Angioplasty of Congenital Anomalies Registry. Am J Cardiol 65:790, 1990 173. Uzun O, Hancock S, Parsons JM, et al: Transcatheter closure of the arterial duct with Cook detachable coils: Early experience. Heart 76:269, 1996 174. Velvis H, Raines KH, Bensky AS, et al: Growth of the right heart after balloon valvuloplasty for critical pulmonary stenosis in the newborn. Am J Cardiol 79:982, 1997 175. Wagner HR, Ellison RC, Keane JF, et al: Clinical course in aortic stenosis. Circulation 56(suppl):I-47, 1977 176. Waldman JD, Schoen FJ, Kirkpatrick SE, et al: Balloon dilatation of porcine bioprosthetic valves in the pulmonary position. Circulation 76:109, 1987 177. Walls JT, Lababidi Z, Curtis JJ et al: Assessment of percutaneous balloon pulmonary and aortic valvuloplasty. J Thorac Cardiovasc Surg 88:352, 1984 178. Ward CJ, Hawker RE, Cooper SG, et al: Minimally invasive management of transposition of the great arteries in the newborn period. Am J Cardiol 69:1321, 1992 179. Wax DF, Rocchini AP: Transcatheter management of venous stenosis. Pediatr Cardiol 19:59, 1998 180. Witsenburg M, The SHK, Bogers AJJC, et al: Balloon angioplasty for aortic recoarctation in children: initial and follow-up results and midterm effect on blood pressure. Br Heart J 70:170, 1993 181. Yetman AT, Nykanen D, McCrindle BW, et al: Balloon angioplasty of recurrent coarctation: A 12-year review. J Am Coll Cardiol 30:811, 1997 31 Figure legends Figure 1. Anterior (left) and lateral (right) right ventriculogram before (upper panel) and after (lower panel) pulmonary valvuloplasty for pulmonary atresia. Note the wide contrast jet through the perforated valve and in the pulmonary artery. Figure 2. Lateral (left and right panels) and anterior (middle panel) aortogram obtained from the transvenous-to-aorta balloon catheter in the aortic arch. The left and middle panels show recoarctation with a typical indentation or “waist”. The right panel shows the same segment immediately after balloon angioplasty with disappearance of the coarctation “waist”. Figure 3. A patient with right pulmonary artery branch stenosis (left panel). The stenotic segment is dilated with stent implantation (right panel). Figure 4. Selective angiogram in a collateral from the Fontan baffle through a Thebesian vein (left panel). Repeat collateral angiogram after delivery of multiple coils, showing complete occlusion (right panel). Figure 5. A 3-dimensional echocardiogram of a double disc umbrella device implanted on atrial septal defect. Panel A shows the device (arrows) in the right atrium and panel B in the left atrium.