* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Montel
Survey
Document related concepts
Microorganism wikipedia , lookup
Bacterial cell structure wikipedia , lookup
Bioremediation of radioactive waste wikipedia , lookup
Disinfectant wikipedia , lookup
Bacterial morphological plasticity wikipedia , lookup
Marine microorganism wikipedia , lookup
Human microbiota wikipedia , lookup
Triclocarban wikipedia , lookup
Listeria monocytogenes wikipedia , lookup
Phospholipid-derived fatty acids wikipedia , lookup
Transcript
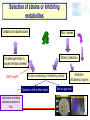
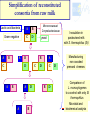
Ecological approaches for managing microbial diversity to improve safety of traditional food. Marie-Christine Montel, Unité de Recherches fromagères INRA Aurillac Example of cheese from experiments performed in WP2A of Truefood project Improvement of microbial safety WP2A Truefood project Task 2A.1 Improvement microbial safety of milks through reduction of mastitis and the use of antibiotics by feeding regimes and other management practices ( ISS, INRA,) Task 2A.2 Management of microbial diversity for inhibiting pathogenic bacteria (L. monocytogenes, S. aureus) in traditional cheeses ( INRA, UL, TUM, DRI) Task 2A.3 Improvement of environmental conditions governing cheese ripening taking account process efficiency and cheese quality (INRA, DRI) Task 2A.4 Development of a bio-preservation (using lactic acid bacteria) for inhibiting food pathogens on pork muscle tissues (ADIV) Why an ecological approach? Because Cheeses and milk are microbial ecosystems – Cheese is a system in which microbial populations (living or biotic part ) live and have relationships between them and with their surrounding environments ( abiotic part) . » Microbial populations interact each other in synergy or antagonism and their life dpends on nutriments, temperature, humididity, oxygen. –The life of these microbes is determinant for the qualities of cheeses. Microbial safety Pathogenic bacteria : • commercialised raw milk cheeses have to comply with EC regulation •Listeria monocytogenes •Salmonella •Staphylococus aureus ( no enterotoxins production ) •Eschercihia coli Opportunist pathogenic bacteria Metabolite productions •Enterotoxins •Mycotoxins •Biogenic amines Above all, great taste, Benefits refer to pleasure and happiness of consumers who require authenticity , traceability, sensorial properties, safety and health values. Why to manage microbial diversity ? To eliminate pathogenic bacteria EC regulation for 4 species Minimise To preserve microbial community: during manufacturing and ripening Increase Risks Benefits Microbial Safety Gustative pleasure Health A challenge for industrial producing Traditional Fermented products (Cheese, sausage…) for conciliating all these aspects and meet all these requirements Our behaviour in managment of microbial diversity for microbial safety must have always in mind the benefits of microbial diversity for cheeses -Healthy aspects? -Contribution in hurdle technology -Diversity of their sensorial properties Management of microbial diversity Elimination of pathogenic micro-organisms and maintain of microbial diversity having an interest through all the process control of raw material : microbial quality of milk, meat... control of microbial dynamics during ripening Tools for tracking and monitoring microbial diversity How to identify and monitor microbial community Molecular Classical Milk , cheeses Picture depending on culture media, cultavibility of strains Identification by phenotypic tests, genomic tests (speciesPCR, 16s or 23s DNAr sequencing) Physical spectra ( FTIR) PCR-SSCP,TGGE, TTGE, DGGE, lH-PCR… Monitor microbial dynamics Picture biased by •Dominant population detected •DNA Extraction •PCR amplification •Coelution in the same peaks Approaches get rich each other What approaches for biopreservation of traditional products ? 1. Selection of strains or metabolites (example : nisine) 2. Understanding microbial ecosystems having antagonist activities • Example of milk and raw milk cheese ecosystems Selection of strains or inhibiting metabolites Validation at industrial scale In vitro screening of inhibitory activity Coculture in milk or other media Identification of inhibiting substances bacteriocins, H 2 O2 , 1 Strains Collections Challenge tests in experimental cheese GAP here!!!! Milks , cheeses Detection of bacteriocin genes Test on agar well Agar diffusion Bioluminescence Selection of strain inhibiting Time consuming and fastidious Frequent fails due to gap between the results obtained in vitro and those obtained in cheeses Consortia with high antilisteria activities by in vitro test but not at the surface of cheeses Low number of strains selected for example in Truefood project able to inhibit and without effect on sensorial properties Strategies by ecological approach Rely on a general principle in ecology indicating that: the whole is more than the sum of each individu as many interactions - synergy, antagonism, competition..- can occur Hypothesis that preservation of the microbial community with the wholeness of its diversity is important for the different functions -inhibition of pathogens, production of aromatic compounds Understanding microbial ecosystem having antilisteria activity Advice for milk production or cheese ripening proposal of consortium Test of simplified microbial consortia in experimental cheese Screening of milks, or cheese surface on experimental cheeses Analysis of the most inhibitory microbial consortia Identification of microbial and biochemical part of the ecosystem Examples of microbial cheese ecosystems with antilisteria activities Microbial consortia from Surfaces of cheeses Smear cheeses: Munster Farm Saint-nectaire cheeses Microbial consortium from Raw milk from Saint-Nectaire area First example consortia of microbes from the surface of Saint-Nectaire Study of Inhibition of Listeria monocytogenes at the surface Antilisteria activities of surfaces of SaintNectaire cheese microbial consortia Comparison of inhibitory effect of consortia (34) selected from farm Saint-Nectaire surface cheeses. (Trials on surface of non cooked pressed 0,5 SN15 SN11 SN26 SN19 SN13 SN9 SN22 SN30 SN24 SN25 SN23 SN12 SN29 SN6 SN8 SN34 SN20 SN28 SN16 SN32 SN2 SN17 SN1 SN35 SN31 SN27 SN3 SN14 SN33 SN18 SN10 SN5 SN4 SN7 cheeses ripened at 7°C during 28 days) Inhibition 0 -0,5 No inhibition -1 -1,5 -2 DLog -2,5 Great diversity in the antilisteria activities High inhibition observed without relation with pH values o 5.9<pH28d<8.4 Selection of one consortium SN15 Stability over storage of the inhibiting effect of SN15 consortia against Listeria monocytogenes ΔLog Lm (ufc/cm²)CFU/cm2 Delta Log [L.monocytogenes] T emps de conservation Time (months) en mois 0 3 4 9 14 16 24 0,00 -0,50 -1,00 -1,50 -2,00 -2,50 -3,00 -3,50 ΔLog Lm = Log (Lm SN15) - Log (Lm control) Microbial consortium SN15 still inhibitory after 24 months storage at -20°C Identification of microbial consortium (SN15) inhibiting L. monocytogenes Identification by phenotypic test, RFLP and 16S A=Lactic acid bacteria (6 species) DNAr sequencing D=Yeasts (4 species) Candida sake Yarrowia lipolytica Debaryomyces hansenii Geotrichum sp. D A D C B C=Gram negative bacteria (3 species) Proteus vulgaris Serratia proteomaculans Pseudomonas fluorescens or syrinqae Lb. casei Lb. curvatus Ln. mesenteroides Carnobacterium mobile Marinilactibacillus psychrotolerans E. faecalis B= Micrococcaceae/ Corynebacteriaceae (7 species) Arthrobacter nicotianae Arthrobacter bergeri Staphylococcus pulvereri Staphylococcus xylosus Brevibacterium linens or casei/ Brevibacterium antiquum Brachybacterium Reconstitution of complex consortium SN15 from the surface Constitution of consortium with 19 strains (one strain by species) inoculated at 2 Log /cm2 at the surface of cheeses Comparison of L. monocytogenes growth at the surface of cheeses with the natural complex or reconstituted consortia 3 yeasts Inhibition 3 Gram negative DLog reconstituted Complex 6 Lactic acid bacteria D 7 Micrococcaceae/ Corynebacteriaceae Complex more inhibitory than the reconstituted Study in progress for understanding why? Second example microbial consortium from raw milk Study Inhibition of L. monocytogenes in the core of cheeses Complex consortium selected in previous study : Millet al, 2006; Saubusse et al, 2007 Composition of Microbial consortium from raw milk having antisteria activty ( Saubusse et al, 2007) Great diversity : 29 microbial species D=Yeasts (5 species) Rhodosporium babjevae Debaryomyces hansenii Candida pseudointermedia C. pararugosa C. deformans D A D C B C=Gram negative bacteria (5 species) Pseudomonas putida Enterobacter amnigenus Acinetobacter sp. Chryseobacterium sp Stenotrophomonas maltophilia A=Lactic acid bacteria (9 species) Lb. casei / Lb. farciminis Lb. curvatus Lb. plantarum Ln. pseudomesenteroides Ln. citreum E. hirae E. Faecalis A. viridans B= Micrococcaceae/ Corynebacteriaceae (10 species) Staph. saprophyticus/ Staph. equorum/ Staph haemolyticusCorynebacterium casei/Coryne. flavescens Arthrobacter nicotianae Brevibacterium linens Exiguobacterium sp. Brachybacterium rhamnosus Macrococcus caseolyticus Simplification of reconstituted consortia from raw milk Lactic acid bacteria Gram negative A B A C A B Micrococcaceae/ Corynebacteriaceae C D yeast B D A B C D A A B B A D C B D D Inoculation in pasteurised milk with S. thermophilus (St) Manufacturing non coocked pressed cheeses Comparison of L. monocytogenes to a control with only St thermophilus Microbial and biochemical analysis Inhibition of L. monocytogenes with simplified consortia in core of cheeses 1,5 ABCD AB AD A ABC ABD BDC BD B 1 0,5 A=Lactic acid bacteria ΔLog Lm 0 B=Micrococcaceae/ Corynebacteriaceae -0,5 -1 C=Gram negative -1,5 D=yeast -2 -2,5 ΔLog Lm = Log (Lm assay) - Log (Lm control) Synergy between lactic acid bacteria and non lactic acid bacteria in the inhibition Loss of inhibition in simplified consortia without lactic acid bacteria Link between [L. monocytogenes] and [organic acids]- [volatil compounds]- pH 5 4 3 1 AB 3-methylbutyric acid 0 pH Butyric acid -1 2-heptanol 2-butanol 2-pentanol ABCD 2 Fact. 2 : 32,90% Low count of listeria L lactate D lactate Acetic acid -2 AD ESTERS -5 KETONES ALDEHYDES Hexanoic acid -4 High count of listeria control ALCOOLS -3 Ethyl formiate Ethyl butanoate B A 2-methyl-propanal butanal 2-hexanone 4-methyl-2-pentanone 2,3-pentanedione 2,3-butanedione 2-butanone -6 -7 -6 -5 -4 -3 -2 -1 0 1 2 3 4 5 6 Active : 51,77% HypothesisFact. on 1the role of acetic acid and D lactate in the inhibition of L. monocytogenes in cheeses Important variation in volatiles profils according to consortia inoculated Inhibition by several hurdles during ripening pH>5.3 Bacteriocins H202 pH <8mg/g <2mg/g Ac. lactic L Ac. lactic D <0.3mg/g Ac. acetic Alcohols Ethyl esters Besides these microbial factors, environnemental factors can also limit the development of pathogenes Temperature, relative humidity during ripening What are the populations involved in the production ? Microbial dynamics studied by culture methods associated with molecular tools Example of microbial balance in cheese with consortium AB the most inhibitory Lactobacillus Nmax Log UFC/g 9 8 7 6 5 19 species inoculated still present Leuconostoc Enteroccoccus BUT Corynebacteriaceae Difficulties to quantifye species Micrococcaceae of this group In conclusion, what applications for traditional fermented food? Arguments for maintaining microbial diversity as a thumb for safety of fermented products ( ex cheese) High inhibitory potentialities of complex microbial ecosytem from the surface of Cheese but application still limited by difficuties to reconsitute it Further studies needed to understand why Scientific data to think about suitable balance between microbial populations in milk Further studies to adapt milk production practices In conclusion, what applications for traditional fermented food? Proposal a simplified microbial consortium still complex associating lactic acid bacteria ( 8 species) and non lactic acid bacteria ( 12 species?) for inhibiting L. monocytogenes in the core of cheese but its industrial use need some improvments Optimise the preparation of the consortium and insure its stability overtime Validate it use at industrial scale WP6 of Truefood Evaluate its effect on sensorial properties Develop rapid methods to quantifye non lactic acid bacteria in cheese Thank you for your attention and coming instead of visiting the museum Partners of WP2A of Truefood project and especially Cécile Callon for her helpful contribution in this presentation Financial support : European Commission under the 6th Framework programme for RTD ( contrat N° Food CT-2006-016264)