* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 3. The Experimental Basis of Quantum Theory
Survey
Document related concepts
Woodward effect wikipedia , lookup
Time in physics wikipedia , lookup
Introduction to gauge theory wikipedia , lookup
Old quantum theory wikipedia , lookup
Hydrogen atom wikipedia , lookup
Density of states wikipedia , lookup
Electromagnetism wikipedia , lookup
Nuclear physics wikipedia , lookup
Renormalization wikipedia , lookup
Conservation of energy wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Photon polarization wikipedia , lookup
Introduction to quantum mechanics wikipedia , lookup
Atomic theory wikipedia , lookup
Wave–particle duality wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Transcript
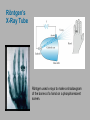
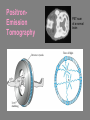
CHAPTER 3 Prelude to Quantum Theory 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Discovery of the X-Ray and the Electron Determination of Electron Charge Line Spectra Quantization Blackbody Radiation Photoelectric Effect X-Ray Production Compton Effect Pair Production and Annihilation Max Karl Ernst Ludwig Planck (1858-1947) We have no right to assume that any physical laws exist, or if they have existed up until now, or that they will continue to exist in a similar manner in the future. An important scientific innovation rarely makes its way by gradually winning over and converting its opponents. What does happen is that the opponents gradually die out. - Max Planck Prof. Rick Trebino, Georgia Tech, www.frog.gatech.edu 3.1: Discovery of the X-Ray and the Electron In the 1890s scientists and engineers were familiar with cathode rays. These rays were generated from one of the metal plates in an evacuated tube with a large electric potential across it. It was surmised that cathode rays had something to do with atoms. It was known that cathode rays could penetrate matter and were deflected by magnetic and electric fields. Observation of X-Rays In 1895, Wilhelm Röntgen studied the effects of cathode rays passing through various materials. He noticed that a phosphorescent screen near the tube glowed during some of these experiments. These new rays were unaffected by magnetic fields and penetrated materials more than cathode rays. He called them x-rays and deduced that they were produced by the cathode rays bombarding the glass walls of his vacuum tube. Wilhelm Röntgen (1845-1923) Röntgen’s X-Ray Tube Röntgen used x-rays to make a shadowgram of the bones of a hand on a phosphorescent screen. Thomson’s Cathode-Ray Experiment In 1897, J.J. Thomson used an evacuated cathode-ray tube to show that the cathode rays were negatively charged particles (electrons) by deflecting them in electric and magnetic fields. Thomson’s Experiment: e/m Thomson’s method of measuring the ratio of the electron’s charge to mass was to send electrons through a region containing an electric field and then also with a magnetic field perpendicular to it. J. J. Thomson (1856-1940) y e v0 B ℓ q x E Calculation of e/m An electron moving through the electric field is accelerated by a force: Fy ma y eE q << 1, so vx ≈ v0 vy t / v0 ayt (eE/m)( /v0) Electron deflection angle: tan(q ) v x v0 v0 unknown To find v0, turn on the magnetic field, which deflects the electron against the electric field force. Use: F eE ev0 B 0 And adjust the magnetic field until the net force is zero. E v0 B v0 E / B Charge-to-mass ratio: tan(q ) e E tan(q ) m B2 eE m ( E/B) 2 3.2: Determination of Electron Charge Millikan’s 1911 oil-drop experiment determined e, hence also m. Robert Andrews Millikan (1868 – 1953) Using an electric field, Millikan suspended tiny charged oil drops. Then he turned off the field and watched them free-fall. Calculation of the oil drop charge The electric field balanced gravity and suspended a charged oil drop: V Fy eE e mdrop g d e mdrop gd / V Turning off the electric field, the drop mass, mdrop, could be determined from Stokes’ relationship of the terminal velocity, vt, to the drop density, , and the air viscosity, h : 2 vt 2 g r / 9h Solving for the drop radius: And using: r 3 h vt / 2 g mdrop 43 r 3 e 18 (h 3 v3t / 2 g )1/2 d / V Millikan experimental results Because a drop could have 1, 2, 3, or more electrons on it, the data looked like this: Thousands of experiments showed that there is a basic quantized electron charge: e = 1.602 x 10-19 C 3.3: Line Spectra Chemical elements were observed to produce unique wavelengths of light when burned or excited in an electrical discharge. Balmer Series In 1885, a school teacher, Johann Balmer, found an empirical formula for the wavelength of the visible hydrogen line spectra in nm: 4 1 1 2 2 364.56nm 2 k 1 (where k = 3,4,5…) Rydberg Equation As more scientists discovered emission lines at infrared and ultraviolet wavelengths, the Balmer series equation was extended to the Rydberg equation: 1 1 RH 2 2 n k 1 RH 1.096776 x 107 m-1 3.5: Blackbody radiation Blackbody radiation is emitted from a hot body. It's anything but black! The name comes from the assumption that the body absorbs at every frequency and hence would look black at low temperature. Blackbody radiation was (and still is) theoretically interesting because the radiation properties of the blackbody are independent of the particular material. 19th-century physicists studied the spectral intensity versus wavelength at various temperatures. Wien’s Displacement Law Wien’s Displacement Law: The maximum of the spectrum shifts to shorter wavelengths as the temperature is increased. But what about the precise shape of the spectral intensity? S(,T) The spectral intensity S(,T) is the total power radiated per unit area per unit wavelength from a blackbody at a given temperature. Lord Rayleigh used the classical theories of electromagnetism and thermodynamics to show that the blackbody spectral distribution should be: S ( , T ) X Spectral Intensity Rayleigh-Jeans Formula 2 ckT 4 Wavelength (nm) It approaches the data at longer wavelengths, but it deviates badly at short wavelengths. This problem for small wavelengths became known as the ultraviolet catastrophe and was one of the outstanding exceptions that classical physics could not explain. Planck’s Radiation Law Planck assumed that the radiation in the cavity was emitted (and absorbed) by some sort of “oscillators.” He used Boltzmann’s statistical methods. But Planck made two modifications to the classical theory: The oscillators (of electromagnetic origin) can only have certain discrete energies, En = nhn, where n is an integer, n is the frequency, and h is a constant: h = 6.6261 × 10−34 J·s—now called Planck’s constant. The oscillators can absorb or emit energy in discrete multiples of the fundamental quantum of energy given by: DE = hn He obtained a formula that fit the blackbody radiation data perfectly: S ( , T ) 2 c 2 h 5 1 e hc / kT 1 Planck’s radiation law 3.6: Photoelectric effect Methods of electron emission: Thermionic emission: Applying heat allows electrons to gain enough energy to escape. Secondary emission: The electron gains enough energy by transfer from another high-speed particle that strikes the material from outside. Field emission: A strong external electric field pulls the electron out of the material. Photoelectric effect: Incident light (electromagnetic radiation) shining on the material transfers energy to the electrons, allowing them to escape. Photo-electric Effect Experimental Setup Photo-electric effect: Classical theory vs. measurements Classical theory predicted that the electrons absorb energy from the beam at a fixed rate. So, for extremely low light intensities, a long time would elapse before any one electron could obtain sufficient energy to escape. But, in actual measurements, the electrons are emitted almost instantly following illumination of the cathode, independent of the intensity of the light. Initial observations by Heinrich Hertz 1887 Photo-electric effect: Classical theory vs. measurements Electron kinetic energy n0 Classical theory also predicted that the kinetic energy of the electrons should increase with the light intensity and not depend on the light frequency. In fact, the kinetic energy of the electrons depends only on the frequency of the light. Also, the number of electrons was proportional to the light intensity. Photo-electric effect: classical theory vs. observations Threshold frequencies Electron kinetic energy n0 Experiments also revealed a threshold frequency of the light, below which no electrons were ejected. The existence of a threshold frequency was completely inexplicable in classical theory. Einstein’s theory: Photons! Flying in the face of a century of observations that light is a wave, Einstein suggested that light is somehow quantized into particles we now call photons. Each photon has energy: E hn where n is the frequency of the light and h is Planck’s constant. Alternatively, because n = w/2: E w where: h / 2 Einstein’s theory Conservation of energy yields: Electron kinetic energy (K) = = Photon energy Potential energy to be overcome K hn f where f is the work function of the metal (potential energy to be overcome before an electron could escape). This simple—but highly unintuitive—idea explained all the properties of the photoelectric effect! Electron kinetic energy n0 Other scientists’ reactions to Einstein’s explanation of the photo-electric effect The data were actually less clear than these slides have implied. Even though it proved his own theory, Planck was skeptical. Millikan spent ten years trying to disprove it, but finally grudgingly published data supporting it in 1916 and won the Nobel Prize in 1923 for it (and his oil-drop experiment). But Millikan still didn’t believe in the photon concept. Einstein finally won the Nobel Prize for this work in 1921 (but wasn’t actually awarded it until 1922). 3.7: Inverse photo-electric effect: Bremsstrahlung x-ray production An energetic electron passing through matter will radiate photons and lose kinetic energy, called Bremsstrahlung. Unlike photons, electrons can lose some of their energy. Since momentum is conserved, the nucleus absorbs very little energy, and it can be ignored. The final energy of the electron is determined from the conservation of energy to be: E f Ei hn e Ei Ef nucleus hn Bremsstrahlung X-ray production: experiment Current in a filament produces electrons by thermionic emission. Accelerated by thousands of volts, the electrons smash into the anode, where they produce x-rays. Their energy before and after: Ei = eV0 and Ef ~ 0 Inverse photoelectric effect Neglect the work function because it’s small compared to the electron energy, which is large here. Conservation of energy requires that the maximum photon energy equal the electron kinetic energy. This yields the Duane-Hunt limit, first found experimentally. The minimum photon wavelength depends only on the accelerating voltage and is the same for all targets. eV0 hn max min hc min hc eV0 Photons also have momentum! Use our expression for the relativistic energy to find the momentum of a photon, which has no mass: E (mc ) p c 2 2 2 Alternatively: When radiation pressure is important: 2 2 E hn h p c c h 2 p k 2 Comet tails (other forces are small) Viking spacecraft (would've missed Mars by 15,000 km) Stellar interiors (resists gravity) 3.8: Compton Effect Ep , pp Photons have energy and momentum: E hc / p h/ e When a photon enters matter, it can interact with one of the electrons. The laws of conservation of energy and momentum apply, as in any elastic collision between two particles. This yields the change in wavelength of the scattered photon, known as the Compton effect: Ee , pe Ep’ , pp ’ h D 1 cos q mc 3.9: Pair Production and Annihilation In 1932, C. D. Anderson observed a positively charged electron (e+) in cosmic radiation. This particle, called a positron, had been predicted to exist several years earlier by Paul Dirac. A photon’s energy can be converted entirely into an electron and a positron in a process called pair production: e e But it doesn’t happen in empty space. Paul Dirac (1902 - 1984) Pair Production in Empty Space E pc hn Energy conservation : E hn E E + The total energies for the electron and positron are: Since me ≠ 0: E E2 p2c 2 me2c 4 E p c This yields a lower limit on the photon energy: Momentum conservation in the direction of propagation (×c) yields: hn p c p c hn pc pc cos(q ) p c cos(q ) This yields an upper limit on the photon energy: hn p c p c A contradiction! Pair production in empty space is impossible! Pair Production in Matter In the presence of matter, the nucleus absorbs some energy and momentum, making the process possible. The photon energy required for pair production in the presence of matter is: hn E E Knucleus hn 2me c 2 1.022 MeV Pair Annihilation A positron passing through matter will likely annihilate with an electron. The electron and positron can form an atom-like configuration first, called positronium. Pair annihilation in empty space produces two photons to conserve momentum. Annihilation near a nucleus can result in a single photon. hv hv Pair annihilation yielding two photons Conservation of momentum: hv1 / c hv2 / c 0 So the two photons will have the same frequency: v1 v2 v Conservation of energy: 2me c 2 hv1 hv2 2hv The two photons from positronium annihilation will move in opposite directions with an energy: hv me c 2 0.511 MeV hv hv PositronEmission Tomography PET scan of a normal brain Radioactivity Radioactive decay is the process by which an unstable atomic nucleus loses energy by emitting particles and/or radiation. The emission is spontaneous—the nucleus decays without collision with another particle. Henri Becquerel 1852-1908 Radioactivity was first discovered in 1896 by the French scientist Henri Becquerel while working on phosphorescent materials. He wrapped a photographic plate in black paper and placed various phosphorescent salts on it. All results were negative until he used uranium salts. The result with these compounds was a deep blackening of the plate. Types of radioactivity There are many types of radioactive emissions. Becquerel, Ernst Rutherford, and Marie and Pierre Curie (~1900) were able to classify them into various types. They included: Marie Skłodowska Curie 1867-1934 a-rays: Helium nuclei (2 protons and 2 neutrons) b-rays: Electrons (or positrons) -rays: -rays N-rays In 1903, Rene Blondlot, of Nancy, France, discovered a new ray that he called the N-ray. N-rays had remarkable properties and could only be seen by dispersing them with an aluminum prism and then by observing weak luminescence in the dark with dark-adapted human eyes. Rene Blondlot 1849–1930 Alas, N-rays didn’t actually exist. After ~300 papers were published on N-rays, American scientist, R.W. Wood, visited Blondlot’s lab and secretly removed the aluminum prism, and Blondlot could still see them. N-rays are probably the greatest case of self-delusion in the history of science.