* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download ppt - SJSU Engineering - San Jose State University
William Flynn Martin wikipedia , lookup
Efficient energy use wikipedia , lookup
Grid energy storage wikipedia , lookup
Open energy system models wikipedia , lookup
100% renewable energy wikipedia , lookup
Energy subsidies wikipedia , lookup
Energy storage wikipedia , lookup
Low-Income Home Energy Assistance Program wikipedia , lookup
Public schemes for energy efficient refurbishment wikipedia , lookup
Zero-energy building wikipedia , lookup
Energy Charter Treaty wikipedia , lookup
Regenerative brake wikipedia , lookup
World energy consumption wikipedia , lookup
Internal energy wikipedia , lookup
International Energy Agency wikipedia , lookup
Alternative energy wikipedia , lookup
Low-carbon economy wikipedia , lookup
Energy returned on energy invested wikipedia , lookup
Energy policy of Australia wikipedia , lookup
Energy policy of Finland wikipedia , lookup
Energy policy of the United Kingdom wikipedia , lookup
Energy harvesting wikipedia , lookup
Conservation of energy wikipedia , lookup
Distributed generation wikipedia , lookup
Negawatt power wikipedia , lookup
Life-cycle greenhouse-gas emissions of energy sources wikipedia , lookup
Energy policy of the European Union wikipedia , lookup
Energy efficiency in transport wikipedia , lookup
Energy in the United Kingdom wikipedia , lookup
Energy efficiency in British housing wikipedia , lookup
United States energy law wikipedia , lookup
Energy applications of nanotechnology wikipedia , lookup
Energy Independence and Security Act of 2007 wikipedia , lookup
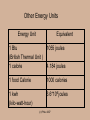
ENGR 10 Lecture on Energy and Power Ping Hsu College of Engineering San Jose State University (c) P.Hsu 2007 What is energy? Energy is what it takes to change the physical state of an “object”. In some cases, energy is released during such a change. Change of the physical state is called ‘work’. (c) P.Hsu 2007 Examples: 0 mph 50 mph 80 mph 65 mph Energy Energy 1 ton 1 ton Gas, air (oxygen) Energy Energy 30ºF Exhaust gas 78ºF H Energy O2 H2O Energy (c) P.Hsu 2007 A mechanical system example: The “work” of changing the state of a mass of m kg from standing still to moving at a speed of v m/sec. requires: 1 2 Energy mv 2 Note: Energy’s unit is Joule. (c) P.Hsu 2007 And… The work of lifting a mass of m kg by h meter requires Energy mgh where g= 9.807, the gravitational constant. (c) P.Hsu 2007 State Change (Work) Required Energy (J) 1068 Creation of the Universe Starting earth moving in orbit 1033 Hiroshima Atomic Bomb Explosion Pushing a 2006 Honda Accord from 0 to 60pmh Hard-hit baseball 1014 (energy release) Lifting an apple by 1 meter 1 Hopping flea (per hop) 10-7 5*105 103 (c) P.Hsu 2007 Other Energy Units Energy Unit Equivalent 1 Btu 1055 joules (British Thermal Unit ) 1 calorie 4.184 joules 1 food Calorie 1000 calories 1 kwh (kilo-watt-hour) 3.6*106 joules (c) P.Hsu 2007 Where does energy come from and where does it go? • Theoretically, the question ‘where does energy come from?’ is the same question ‘where does the universe come from? • In a more practical term, energy is ‘stored’ in various forms around us. Energy Content (J) Gallon of gasoline 1.3 x 108 Pound of coal 1.6 x 107 Candy bar 2 x 105 AA battery 103 A passenger car at 60mph 5 x 105 (c) P.Hsu 2007 Where does Energy go? Energy ‘used’ in the physical state transformation process is ‘contained’ in the new physical state of the objects. Example: It takes 5x105J of energy to move a Honda Accord from 0 to 60mph. This amount of energy is contained in this moving car. This energy is called Kinetic Energy. Example: The energy that was used in lifting a weight is ‘stored’ in the new state (a higher elevation) of the weight. This energy is called Potential Energy. (c) P.Hsu 2007 Example: The energy that was used in heating up a room (from burning wood, e.g.) is stored in the warn air in the room. Example: The energy that was used for bringing you from home to school this morning is contained in your car parked in the garage right now. Correct? (c) P.Hsu 2007 Can energy be destroyed? No! Conservation of Energy Energy never vanishes. Energy only changes into different forms. • ‘Efficiency’ of a energy conversion is the ratio between the part of the energy that caused the desired effect and the total energy used, i.e., in this example, = Gas, air Exhaust gas Heated engine Stirred up air 0 mph Kinetic energy of the car Total energy released from gas combustion (c) P.Hsu 2007 50 mph IF energy never disappears, why do we have energy crisis? Energy Conservation Car Never Needs Gas! Some form of energy is difficult or even impossible to transform efficiently to a re-useable form. The energy in the exhaust gas from a car engine or heating of the brake are such form of energy. When energy is converted into such a form, it is essentially lost, from a practical point of view. (c) P.Hsu 2007 How difficult is it to convert energy from one form to another (a more useful) form? Very Easy: Burning wood, coal, fossil fuel, potential energy stored in the water in a reservoir, nuclear reaction, energy stored in a battery, etc. Not difficult but cost more: Solar, wind (kinetic energy of the air mass), ocean current (kinetic energy of water). Impossible to transform efficiently to a ‘useful’ form: Energy in the exhaust gas from a car engine. Energy in the warm ocean water in a tropical region. (c) P.Hsu 2007 Energy Conversion Machines Some energy conversion can be done by a natural process such as burning wood for heat. To better suit our need, we build machines to facilitate, manage, and control the energy conversion. (c) P.Hsu 2007 For example, in a car’s engine: – The spark plugs initiate the combustion which release energy form the gasoline. – The cylinders and pistons transform the expansion force of the combusted fuel/air to mechanical rotational force (torque). – The cooling systems takes the heat energy ( a byproduct) away from the engine. (c) P.Hsu 2007 Gas, air Exhaust gas 50 mph 0 mph 25ºC Break 200ºC Break Heated engine Stirred up air 0 mph 50 mph (c) P.Hsu 2007 Fast moving air (wind) 30ºF Slower moving air 78ºF (c) P.Hsu 2007 Key Concepts • Energy is what it takes to (or releases from) change the physical state of an “object”. • Energy cannot be ‘created’ or ‘destroyed’. • Efficiency is the ratio between the part of output energy that is beneficial to us and the total used energy • We build machines to ‘manage’ energy conversion. • While energy cannot be destroyed, once it is transformed into a certain form (heat, often the case), it is basically lost. (c) P.Hsu 2007 What is power? (c) P.Hsu 2007 It takes time to convert energy from one form to another! ‘Power’ is a measure of how fast energy is converted. The unit of power is WATT. 1 watt = 1 Joule/second (c) P.Hsu 2007 Example: 1500J of electrical energy per second 1500J heat energy per section This heater converts 1500J of electrical energy into heat energy per second. This electric heater’s power rating is 1500 J/s or watt. (c) P.Hsu 2007 Analogy: 1500 gallon per minute This pump is rated 1500 g/m. 1500 gallon per minute (c) P.Hsu 2007 Other Units of Power horse power (HP) 1HP = 746 Watts (Used mostly for mechanical systems) Kilo-Watt (KW) 1KW = 1000 watts BTU per Hour (BTUH) 1BTUH = ? watts (Used mostly for thermal systems) (c) P.Hsu 2007 Back to Energy for a moment Kilo-Watt-Hour (kwh) is a unit of energy. Since 1w=1Joule/second, 1 kwh=1000 (J/s) * 3600 (s) = 3.6*106 joules You are charged by PG&E monthly by the amount of energy in kwh. You are paying about 25 cents for 1kwh of energy these days. (c) P.Hsu 2007 PG&E: Power costs to increase in '08 David R. Baker, Chronicle Staff Writer 9/1/07 According to PG&E's estimates, homeowners using 560 kilowatt-hours per month would see their electricity bills rise by 28 cents, to $74.54. Those using 1,000 kilowatt-hours per month would spend $2.70 more on their monthly electric bills, for a total of $199.83. (c) P.Hsu 2007 Energy vs. Power The information about a certain amount of energy (J) does not involve a sense of time. (Analogy: 100 gallon tank ) Power (J/s=w), on the other hand, tells how fast the energy being converted. (Analogy: 2 gallon/sec. pump) (c) P.Hsu 2007 More Analogy It make sense to say: Tank •The tank has 500 gallon of water. •The pump is pumping 10 gallon per Pump minute. It does not make sense to say: •The tank has 10 gallon per minute of water. •The pump is pumping 500 gallon. (c) P.Hsu 2007 (Q1) Which of the following heaters can heat up a gallon of water to 90oC. (A) 5W heater (B) 90W heater (C) 100W heater (D) All of the above (c) P.Hsu 2007 (Q2) Which of the following heaters can heat up a gallon of water from 10oC to 90oC faster? (A) 5W heater (B) 90W heater (C) 100W heater (D) All heaters above will take the same amount of time. (c) P.Hsu 2007 (Q3) Which equipment will cost you more to run? (A) a 5W equipment (B) a 50W equipment (C) a 100W equipment (D) Insufficient information. (c) P.Hsu 2007 (Q4) John and Peter each has $1000 in his piggybank. They decided to use the money to buy music CDs. Each CD cost exactly $5. John took $20 out of the piggybank each week and Peter, $50. Who will have the most CDs? (A) John (B) Peter (C) Same (D) Insufficient information. (c) P.Hsu 2007 (Q5) Both your and your neighbor's basement are filled with water from the storm. You are given a 5HP gasoline power pump and your neighbor, a 10HP pump. Each of you is given only 1 gallon of gasoline. If both pumps have the same efficiency, which one can pump more water out of the basement ? (A) You (B) Your neighbor (C) Same (D) Insufficient information. (c) P.Hsu 2007