* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Structure, function and mechanism of G
Survey
Document related concepts
Model lipid bilayer wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
SNARE (protein) wikipedia , lookup
Theories of general anaesthetic action wikipedia , lookup
Cytokinesis wikipedia , lookup
P-type ATPase wikipedia , lookup
Rho family of GTPases wikipedia , lookup
Cell membrane wikipedia , lookup
Endomembrane system wikipedia , lookup
Gap junction wikipedia , lookup
Signal transduction wikipedia , lookup
Transcript
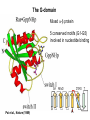
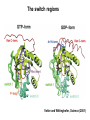
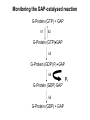
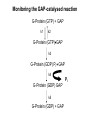
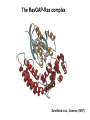
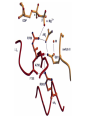
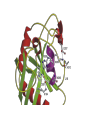
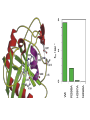
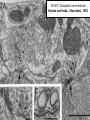
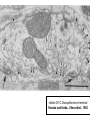
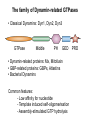
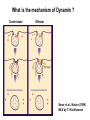
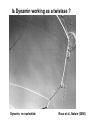
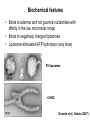
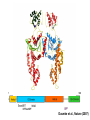
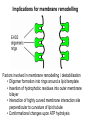
Structure, function and mechanisms of G-Proteins Oliver Daumke MDC-Berlin, House 31.2 (Flachbau), R0225 [email protected] 1994 Nobel Prize in Medicine, Alfred Gilman and Martin Rodbell, for their „discovery of G-Proteins and the role of these proteins in signal transduction in cells.“ G-Protein = Guanine-nucleotide binding protein (GNBD) Guanine 6 1 Anhydride Ester 7 5 2 4 9 8 3 5 Guanosine α 4 1 3 2 Phosphates Ribose Guanosine-triphosphate - GTP G-Protein families • Heterotrimeric G-Proteins (Transducin, Gi, Gq …), in 7-TM receptor signalling • Initiation, elongation, termination factors in protein synthesis (IF1, EF-Tu, EF-TS) • Signal recognition particle (SRP) and its receptor, translocation of nascent polypeptide chains in the ER • Ras-like GTPases (Ras, Rap, Rho, Ran, Rab, Arf, Arl, Sar), molecular switches in signal transduction • Dynamin superfamily of GTPases, remodelling of membranes + 60 further distinct families Leipe et al., JMB (2002) The G-domain Mixed - protein 5 conserved motifs (G1-G5) involved in nucleotide binding Pai et al., Nature (1989) Ras-like G-Proteins are molecular switches To allow switch function: high affinity for nucleotide required pMol Effector: Interacts stably with the GTP-bound form GEF: Guanine nucleotide Exchange Factor GAP: GTPase Activating Protein The switch regions Vetter and Wittinghofer, Science (2001) The GTPase reaction • Intrinsic GTPase rates of small G-Proteins are slow (range: kcat=10-2 - 10-3 min-1) • SN2 nucleophilic attack with trigonal bipyramidal transition state • Phosphate hydrolysis reaction is thermodynamically highly favourable but kinetically very slow (Westheimer FH (1987), Why nature chose phosphates, Science 235, 1173-1178) Enzymatic strategies for GTP hydrolysis 1) Counteracting of negative charge at phosphates - P-loop (GxxxxGKS), hydrogen bonds and lysine - Mg2+ ion, essential for nucleotide binding and hydrolysis - catalytic arginine (and lysine residues) 2) Positioning of attacking nucleophile - catalytic glutamine Non-hydrolysable GTP analogues Abbreviations GTP--S GMPPCP GMPPNP Transition state mimicks of GTP hydrolysis GTPase Activating Proteins • Accelerate intrinsic GTPase by a factor of 105 – 106 • Ras, Rap, Rho, Rab, Ran have completely unrelated GAPs • High affinity binding to the GTP-bound form, low affinity interaction with the GDP-bound form • Mechanism of GTP hydrolysis ? Monitoring the GAP-catalysed reaction G-Protein (GTP) + GAP k1 k2 G-Protein (GTP)GAP k3 G-Protein (GDP) Pi GAP k4 G-Protein (GDP) GAP k5 G-Protein (GDP) + GAP Pi Multiple-turnover assays • Monitors several rounds of GAP catalysed G-Protein (GTP) hydrolysis • G-Protein (GTP) as substrate, in excess, e.g. 200 µM • GAP in catalytic amounts, e.g. 100 nM • Determine initial rates of GTP hydrolysis by – HPLC (ratio GDP, GTP) – Thin layer chromatography using radioactively labelled GTP – Phosphate release (colorimetric assay, radioactive assays) • Vary concentration of G-Protein to determine Michaelis-Menten parameters (KM, kcat) Monitoring the GAP-catalysed reaction G-Protein (GTP) + GAP k1 k2 G-Protein (GTP)GAP k3 G-Protein (GDP) Pi GAP k4 G-Protein (GDP) GAP k5 G-Protein (GDP) + GAP Pi Single-turnover assays • Analysis of a single cycle of GTP hydrolysis • Often monitored by fluorescence stopped-flow • Typically 1 – 2 µM fluorescently labelled G-Protein (GTP) in one cell, excess of GAP in the other cell • Vary concentration of GAP → multiparameter fit allows determination of k1, k2, KD, … The mechanism of RasGAP Scheffzek et al., Nature (1996) Fluorescence stopped-flow to monitor the GAP reaction Ras(mantGTP) vs. RasGAP Fluorescence increase: complex formation Fluorescence decrease: GTP hydrolysis Ahmadian et al., Nature Structure Biology (1997) An arginine residue in RasGAPs is essential for GAP activity Ras(mantGTP) vs. RasGAP Ahmadian et al., Nature Structure Biology (1997) AlF3 promotes formation of a transition state complex Mittal et al., Science (1994) The RasGAP-Ras complex Scheffzek et al., Science (1997) Rap1 • Involved in various signalling pathways, e.g. integrin activation • close Ras homologue BUT: No catalytic glutamine residue • own set of GAPs with no sequence homology to RasGAPs 180000 160000 140000 counts 120000 100000 80000 60000 40000 20000 0 0 100 200 300 sec 100 nM RapGAP 800 µM Rap1(GTP) 400 500 Rap1GAP stimulates intrinsic Rap1 reaction 100.000 fold kcat= 6 s-1 Km = 50 µM Brinkmann et al., JBC (2001) No arginine finger is involved in catalysis Brinkmann et al, JBC (2001) The Rap1GAP Dimer Daumke et al., Nature (2004) The catalytic domain of Rap1GAP has a G-domain fold Ras Rap1GAP cat Rap1-Rap1GAP reaction followed by fluorescence stopped-flow R286 is not essential for the GAP reaction His287 is involved in binding to Rap1 Rap1GAP provides a catalytic Asn, the „Asn thumb“, for catalysis Daumke et al., Nature (2004) Asn290 is a purely catalytic residue and not involved in binding to Rap1 Kd = 4 M Rap1GAP-Rap1 complex indicates that Asn thumb positions attacking water molecule Scrima et al., EMBOJ (2008) The Dynamin-family of GTPases The shibire fly Bing Zhang, UT Austin Wt 30°C Drosophila nerve terminal Kosaka and Ikeda, J Neurobiol., 1982 shibire 30°C Drosophila nerve terminal Kosaka and Ikeda, J Neurobiol., 1982 The family of Dynamin-related GTPases • Classical Dynamins: Dyn1, Dyn2, Dyn3 GTPase Middle PH GED • Dynamin-related proteins: Mx, Mitofusin • GBP-related proteins: GBPs, Atlastins • Bacterial Dynamins Common features: - Low affinity for nucleotide - Template induced self-oligomerisation - Assembly-stimulated GTP hydrolysis PRD 1000 x stimulation of Dynamin‘s GTPase reaction by lipid tubule binding Stowell et al., Nat Cell Biol (1999) What is the mechanism of Dynamin ? Constrictase Effector Sever et al., Nature (1999) N&V by T. Kirchhausen Is Dynamin a popase ? No Dynamin GTP--S GDP Stowell et al., Nat Cell Biol (1999) www.endocytosis.org Is Dynamin working as a twistase ? Dynamin, no nucleotide Roux et al., Nature (2006) Dynamin, addition GTP Roux et al., Nature (2006) Biotin-Dynamin streptavidin – polysterene bead Dynamin, addition GTP Roux et al., Nature (2006) The EHD family • EHD = Eps15 homology domain containing protein • Highly conserved in all higher eukaryotes, but not in yeast and bacteria • Four paralogues in human, 70 - 80% amino acid identity Biochemical features • Binds to adenine and not guanine nucleotides with affinity in the low micromolar range • Binds to negatively charged liposomes • Liposome-stimulated ATP hydrolysis (very slow) PS liposomes + EHD2 Daumke et al., Nature (2007) Daumke et al., Nature (2007) Lipid binding site of EHD2 Implications for membrane remodelling Factors involved in membrane remodelling / destabilisation • Oligomer formation into rings around a lipid template • Insertion of hydrophobic residues into outer membrane bilayer • Interaction of highly curved membrane interaction site perpendicular to curvature of lipid tubule • Conformational changes upon ATP hydrolysis Acknowledgements / References • Alfred Wittinghofer Vetter and Wittinghofer „The Guanine nucleotide binding switch in three dimensions.“ Science (2001) Bos, Rehmann, Wittinghofer „GEFs and GAPs critical elements in the control of G-Proteins.“ Cell (2007) A. Wittinghofer, H. Waldmann. „Ras - A molecular switch involved in tumor formation.“ Angew. Chem. Int. Ed. (2000) Scheffzek, Ahmadian, Kabsch, Wiesmuller, Lautwein, Schmitz & Wittinghofer „The Ras-RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants.” Science (1997) • Harvey McMahon (www.endocytosis.org) Praefcke, McMahon, „The dynamin superfamily: universal membrane tubulation and fission molecules?” Nat Rev Mol Cell Biology (2004) McMahon, Gallop, „Membrane curvature and mechanisms of dynamic cell membrane remodelling”, Nature (2005)