* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Transcatheter Closure of Atrial Septal Defect in Ebstein`s Anomaly
Survey
Document related concepts
Cardiac contractility modulation wikipedia , lookup
Electrocardiography wikipedia , lookup
Heart failure wikipedia , lookup
Coronary artery disease wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Echocardiography wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Cardiac surgery wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
Transcript
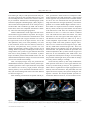
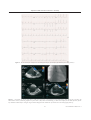
Case Reports Acta Cardiol Sin 2008;24:51-5 Transcatheter Closure of Atrial Septal Defect in Ebstein’s Anomaly Using the Amplatzer Septal Occluder Ming-Chien Wu, Chih-Yuan Fang, Mien-Cheng Chen and Morgan Fu Ebstein’s anomaly is frequently associated with atrial septal defect (ASD). This interatrial communication also contributes to systemic desaturation, systemic embolization, pulmonary hypertension, and right-side heart failure. We describe a case of a 25-year-old lady who had central cyanosis and exercise intolerance due to Ebstein’s anomaly with ASD. These manifestations markedly improved following closure of the ASD using an Amplatzer septal occluder. In the literature, transcatheter closure device for ASD is safe and effective, however, this has rarely been reported regarding Ebstein’s anomaly. Key Words: Ebstein’s anomaly · Atrial septal defect · Amplatzer septal occluder INTRODUCTION overloads. Closure of interatrial communication might prevent, improve or resolve these conditions. In the current era, transcatheter closure can be performed safely and successfully.7 Percutaneous closure of Ebstein’s anomaly-associated ASD is rarely reported.8 We report our experience of transcatheter closure of ASD using Amplatzer septal occluder in a case with Ebstein’s anomaly. In patients with Ebstein’s anomaly, a portion of the right ventricle (RV) is atrialized, and the functional RV is small. In addition, most patients have some interatrial communication [atrial septal defect (ASD) or patent foramen ovale (PFO)] through which right-to-left shunting may occur.1 The clinical presentation varies with age. Rhythm abnormality is the most common presentation in adolescents and adults with Ebstein’s anomaly,2 and is the major cause of significant morbidity and sudden death.3-6 In infants and children, right-to-left atrial shunting can result in cyanosis. The interatrial communication also contributes to late right ventricular failure, arrhythmia, and pulmonary hypertension due to right ventricle CASE REPORT A 25-year-old lady had uncertain diagnosed heart disease since the age of six. She had exercise intolerance, easy fatigue and cyanosis of nail bed since childhood. However, she hadn’t experienced orthopnea, paroxysmal nocturnal dyspnea, or leg edema before. She suffered from sudden onset of dyspnea and palpitation on the morning of admission. She denied fever, chest pain, diaphoresis, hemoptysis and tarry stool passage. Auscultation revealed a grade II/VI pansystolic murmur over the left lower sternal border. There was no hepatomegaly or leg edema, but cyanosis with clubbing of fingers and toes was observed. Initial evaluation at emergency department revealed severe hypoxia (the oxygen saturation was 67.9% by ar- Received: July 23, 2007 Accepted: September 10, 2007 Division of Cardiology, Chang Gung Memorial Hospital-Kaohsiung Medical Center, Chang Gung University College of Medicine, Taiwan. Address correspondence and reprint requests to: Dr. Chih-Yuan Fang, Division of Cardiology, Chang Gung Memorial Hospital-Kaohsiung Medical Center, 123, Ta Pei Road., Niao Sung Hsiang, Kaohsiung Hsien 833, Taiwan, R.O.C. Tel: 886-7-731-7123 ext. 2363; Fax: 886-7- 731-7123 ext. 2355; E-mail: [email protected] 51 Acta Cardiol Sin 2008;24:51-5 Ming-Chien Wu et al. rents, percutaneous ASD closure was attempted. Under general anesthesia and TEE monitoring, a right femoral venous approach was performed with a 6-French sheath. A J-curve wire was advanced from the right atrium through the ASD to the left upper pulmonary vein with the guidance of a 6-French Judkins Right 4 catheter. The venous sheath was replaced with an 8-French Amplatzer delivery sheath, and the tip of the catheter was advanced to the left atrium. Under TEE, one secundum-type ASD measured 11.4 mm ´ 11.5 mm at 42° and 86° of biatrial view with bidirectional shunt and another tiny ASD measured 1.82 mm with right-to-left shunt (Figure 3A). The distance between these two ASDs was 11 mm. A 15-mm Amplatzer septal occluder was well deployed to the larger ASD under TEE guidance (Figure 3B-C). There was no residual shunt through the larger ASD. The tiny ASD shunt remained (Figure 3D). There was neither damage to the adjacent valve nor obstruction of the blood flow. The patient tolerated the procedure well without complication. Her systemic arterial oxygen rose from 81% to 97% immediately after ASD closure, and no significant hemodynamic changes occurred during the procedure. Mean right atrial pressure did not change obviously (from 6 mmHg to 7 mmHg). On the next day, follow-up TTE study confirmed the stable position of the device. The oxygen saturation was 95.9% by arterial blood gas analysis. No significant residual shunt or significant tricuspid regurgitation were found by TTE one month later. The patient did well with improved exercise capacity and cyanosis during the subsequent 6 months. No tachyarrhythmia episodes occurred after ablation. However, we will continue to mo- terial blood gas analysis) and supraventricular tachycardia with hypotension that needed cardioversion. The electrocardiogram after cardioversion showed ventricular pre-excitation. Transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) (Figure 1) showed downward displacement of the tricuspid valve, atrialized RV and secundum-type ASD with bi-directional shunt, which was compatible with Ebstein’s anomaly. There was mild tricuspid regurgitation. Cardiac catheterization of the right-side heart showed left atrium oxygen saturation step-down. The oxygenation series disclosed interatrial communication with bidirectional shunt (the oxygen saturation was 59% in the right atrium, 59% in the right ventricle, 59% in the main pulmonary artery, 97% in the pulmonary vein, 81% in the left atrium, 84% in the left ventricle, and 81% in the aorta). The pulmonary artery pressure was 14/7 mmHg, and pulmonary capillary wedge pressure was 10 mmHg. While temporally occluding the ASD using a sizing balloon, the systemic oxygen saturation increased from 81% to 98% gradually over 10 minutes. Cyanosis improved and the patient tolerated ASD closure well. No deteriorated hemodynamic changes occurred. The blood pressure was around 102/64 mmHg. Later, electrophysiologic study was performed for Wolff-Parkinson-White syndrome, and the patient experienced hypotension when supraventricular tachycardia was induced. Two accessory (right posterior and right lateral) pathways were identified and ablated successfully (the electrocardiograms before and after ablation are shown in Figure 2). After obtaining consent from the patient and her pa- Figure 1. (A) Four-chamber view of transthoracic echocardiography revealed apical displacement of the tricuspid valve and atrialized right ventricle. (B) Left-to-right shunt from the atrial septal defect (ASD) by transesophageal echocardiography. (C) Right-to-left shunt from the ASD. Acta Cardiol Sin 2008;24:51-5 52 Amplatzer ASD Occluder in Ebstein’s Anomaly Figure 2. The 12-lead surface complete electrocardiograms before (A) and after (B) ablation of accessory pathways. Figure 3. (A) Another tiny atrial septal defect (ASD) was detected by transesophageal echocardiography (TEE) during the procedure. (B) Transcatheter closure of ASD using Amplatzer septal occluder (ASO) under TEE monitoring (C) ASO was well deployed with stable position. (D) The tiny ASD shunt remained after closing the larger ASD but disappeared one month later by transthoracic echocardiography follow-up. 53 Acta Cardiol Sin 2008;24:51-5 Ming-Chien Wu et al. tion immediately, prevent desaturation during exercise, and improve daily work capacity. 10 Since the transcatheter closure of ASD is feasible by the Amplatzer septal occluder,12 it provides a non-surgical method to mitigate the patient’s symptoms. Agnoletti et al 8 reported the successful transcatheter closure of interatrial shunt in patients with Ebstein’s anomaly. All of these patients had the minor form of Ebstein’s disease, with only symptoms of desaturation. Only one patient had age similar to our patient’s. None had significant changes of tricuspid valve regurgitation after ASD closure. The closure of the interatrial communication is not thought to prevent a possible surgical option in the future. Indeed, the risk of repeated stroke is considered important.8 In conclusion, patients with Ebstein’s anomaly often develop systemic desaturation and risk of systemic embolization due to right-to-left shunting. Before deterioration of right-side heart function and tricuspid regurgitation, early intervention to repair the interatrial communication might improve outcomes. In our report, transcatheter Amplatzer septal occluder for Ebstein’s anomaly concomitant ASD is a feasible and safe way. The patient got significant improvement of cyanosis and exercise intolerance after closure of interatrial shunting. Although the long-tern outcome may need more time to observe, the immediate and short-term results are quite encouraging. nitor this patient for long-term outcome. DISCUSSION Ebstein’s anomaly is an unusual congenital heart disease with variable clinical presentation. The characteristic anatomic feature is downward displacement and adherence of dysplastic septal and posterior tricuspid leaflets into the right ventricle. The right ventricle is divided into a so-called atrialized chamber and a functionally reduced right ventricle. 1-2 Patients with Ebstein’s anomaly have a high potential for developing arrhythmia. Most of these tachyarrhythmias are based on accessory pathways located along the anomalous atrioventricular valve.6 A single accessory pathway was present in 52% and multiple accessory pathways in 29% of cases. Ablation for accessory pathway has initial success in 79~89% of cases; however, the recurrence rate is 29~32%.9 Our patient had Ebstein’s anomaly without any treatment till adolescence. She had only mild symptoms with exercise intolerance and cyanosis. So, she didn’t have heart failure sign in the past. She was admitted because of newly experienced supraventricular tachycardia. After electrophysiologic study, catheter ablation was performed for the tachyarrhythmia successfully. The further therapeutic consideration for this patient is how to improve her life quality. In fact, the right-to-left shunting in the congenital heart disease is associated with two major complications: systemic embolization and desaturation. The cerebral events are mostly in the presence of a right-to-left shunting through the atrial septum. Percutaneous closure of patent foramen ovale is considered at least as effective as medical treatment in patients with cryptogenic stroke and recurrent cerebrovascular events.10 Chen et al reported that earlier intervention in Ebstein’s anomaly should be considered before the development of significant deterioration in right-side heart function and tricuspid regurgitation. The early results for surgical repair of Ebstein’s anomaly are quite good.11 The development of symptomatic dyspnea or cyanosis is related to right-to-left shunting or decline in right ventricular function. Closure of such shunt will be able to promote the increase of systemic oxygen saturaActa Cardiol Sin 2008;24:51-5 REFERENCES 1. Brickner EM, Hillis LD, Lange RA. Congenital heart disease in adults: second of two parts. N Engl J Med 2000;342:334-42. 2. Celermajer DS, Bull C, Till JA, Cullen S, Vassillikos V, Sullivan ID, et al. Ebstein’s anomaly: presentation and outcome from fetus to adult. J Am Coll Cardiol 1994;23:170-6. 3. Lo H, Lin F, Jong Y, Tseng Y, Wu T. Ebstein’s anomaly with ventricular tachycardia: evidence for the arrhythmogenic role of the atrialized ventricle. Am Heart J 1989;117:959-62. 4. Cappato R, Schluter M, Weiss C, Antz M, Kopschyk DH, Hofman T, et al. Radiofrequency current catheter ablation of accessory atrioventricular pathways in Ebstein’s anomaly. Circulation 1996;94:376-83. 5. Oh J, Holmes DR, Hayes DL, Porter CJ, Danielson GK. Cardiac arrhythmias in patients with surgical repair of Ebstein’s anomaly. J Am Coll Cardiol 1985;6:1351-7. 6. Hebe J. Ebstein’s anomaly in adults. Arrhythmias: diagnosis and therapeutic approach. Thorac Cardiovasc Surg 2000;48(4):214-9. 54 Amplatzer ASD Occluder in Ebstein’s Anomaly 7. Butera G, De Rosa G, Chessa M, et al. Transcatheter closure of atrial septal defect in young children: results and follow-up. J Am Coll Cardiol 2003;42:241-5. 8. Agnoletti G, Boudjemline Y, Ou P, Bonnet D, Sidi D. Right to left shunt through interatrial septal defects in patients with congenital heart disease: results of interventional closure. Heart 2006; 92(6):827-31. 9. Reich JD, Auld D, Hulse E, Sullivan K, Campbell R. The Pediatric Radiofrequency Ablation Registry’s experience with Ebstein’s anomaly. Pediatric Electrophysiology Society. J Cardiovasc Electrophysiol 1998;9(12):1370-7. 10. Widecker S, Wahl A, Nedeltchev K, et al. Comparison of medical treatment with percutaneous closure of patent foramen ovale in patients with cryptogenic stroke. J Am Coll Cardiol 2004; 44:750-8. 11. Chen JM, Mosca RS, Altmann K, et al. Early and medium-term results for repair of Ebstein anomaly. J Thorac Cardiovasc Surg 2004;127:990-8. 12. Masura J, Gavora P, Formanek A, Hijazi ZM. Transcatheter closure of secundum atrial septal defects using the new self-centering amplatzer septal occluder: initial human experience. Cathet Cardiovasc Diagn 1997;42(4):388-93. 55 Acta Cardiol Sin 2008;24:51-5








![Percutaneous interventions in CHD [1]Shunt lesions](http://s1.studyres.com/store/data/002863054_1-7d67b4fe57fdc0f6dc9fed277d528ca5-150x150.png)


